Page 352 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 352
328 15 New Applications of Transketolase: Cascade Reactions for Assay Development
25 TK yst 1.2 TK yst
TK eco TK eco
D469E
D469E 1.0 TK eco
20 TK eco
0.8
U mg −1 15 U mg −1 0.6
10
0.4
5
0.2
0 0.0
200 mM L-lyxose
200 mM 3-hydroxypropanal
50 mM D,L-2,4-dihydroxybutanal
200 mM 2-deoxy-D-ribose
200 mM propanal
50 mM D,L-glyceraldehyde
200 mM D-glucose
50 mM methanal 50 mM D-glyceraldehyde 50 mM D-erythrose 200 mM ethanal 200 mM D-ribose 200 mM L-arabinose 200 mM L-mannose 200 mM 4-hydroxybutanal
50 mM glycolaldehyde
50 mM L-threose
200 mM D-allose
200 mM D-gulose
200 mM (R)-3,4-dihydroxybutanal
200 mM D-xylose
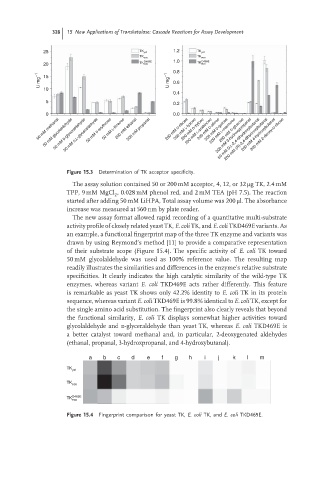
Figure 15.3 Determination of TK acceptor specificity.
The assay solution contained 50 or 200 mM acceptor, 4, 12, or 32 μg TK, 2.4 mM
TPP, 9 mM MgCl , 0.028 mM phenol red, and 2 mM TEA (pH 7.5). The reaction
2
started after adding 50 mM LiHPA, Total assay volume was 200 μl. The absorbance
increase was measured at 560 nm by plate reader.
The new assay format allowed rapid recording of a quantitative multi-substrate
activity profile of closely related yeast TK, E. coli TK, and E. coli TKD469E variants. As
an example, a functional fingerprint map of the three TK enzyme and variants was
drawn by using Reymond’s method [11] to provide a comparative representation
of their substrate scope (Figure 15.4). The specific activity of E. coli TK toward
50 mM glycolaldehyde was used as 100% reference value. The resulting map
readily illustrates the similarities and differences in the enzyme’s relative substrate
specificities. It clearly indicates the high catalytic similarity of the wild-type TK
enzymes, whereas variant E. coli TKD469E acts rather differently. This feature
is remarkable as yeast TK shows only 42.2% identity to E. coli TK in its protein
sequence, whereas variant E. coli TKD469E is 99.8% identical to E. coli TK, except for
the single amino acid substitution. The fingerprint also clearly reveals that beyond
the functional similarity, E. coli TK displays somewhat higher activities toward
glycolaldehyde and d-glyceraldehyde than yeast TK, whereas E. coli TKD469E is
a better catalyst toward methanal and, in particular, 2-deoxygenated aldehydes
(ethanal, propanal, 3-hydroxpropanal, and 4-hydroxybutanal).
a b c d e f g h i j k l m
TK yst
TK
eco
TK D469E
eco
Figure 15.4 Fingerprint comparison for yeast TK, E. coli TK, and E. coli TKD469E.