Page 344 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 344
320 15 New Applications of Transketolase: Cascade Reactions for Assay Development
stereochemical probes for measuring wild-type or altered TK activity from enzyme
variants with improved or new properties. The assay was validated using wild-type
TK and probe 1 in the presence of d-R5P as the acceptor substrate.
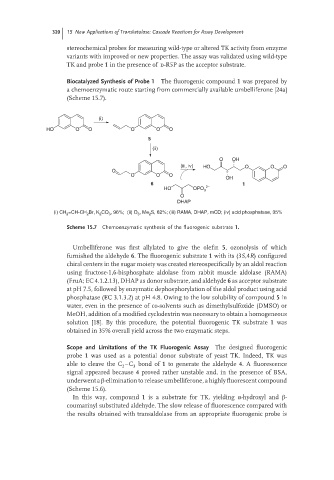
Biocatalyzed Synthesis of Probe 1 The fluorogenic compound 1 was prepared by
a chemoenzymatic route starting from commercially available umbelliferone [24a]
(Scheme 15.7).
(i)
HO O O O O O
5
(ii)
O OH
(iii, iv) HO O O O
O
O O O
OH
6 2− 1
HO OPO 3
O
DHAP
(i) CH =CH-CH Br, K CO , 96%; (ii) O , Me S, 62%; (iii) RAMA, DHAP, mCD; (iv) acid phosphatase, 35%
2
2
2
3
3
2
Scheme 15.7 Chemoenzymatic synthesis of the fluorogenic substrate 1.
Umbelliferone was first allylated to give the olefin 5,ozonolysisofwhich
furnished the aldehyde 6. The fluorogenic substrate 1 with its (3S,4R) configured
chiral centers in the sugar moiety was created stereospecifically by an aldol reaction
using fructose-1,6-bisphosphate aldolase from rabbit muscle aldolase (RAMA)
(FruA; EC 4.1.2.13), DHAP as donor substrate, and aldehyde 6 as acceptor substrate
at pH 7.5, followed by enzymatic dephosphorylation of the aldol product using acid
phosphatase (EC 3.1.3.2) at pH 4.8. Owing to the low solubility of compound 5 in
water, even in the presence of co-solvents such as dimethylsulfoxide (DMSO) or
MeOH, addition of a modified cyclodextrin was necessary to obtain a homogeneous
solution [18]. By this procedure, the potential fluorogenic TK substrate 1 was
obtained in 35% overall yield across the two enzymatic steps.
Scope and Limitations of the TK Fluorogenic Assay The designed fluorogenic
probe 1 was used as a potential donor substrate of yeast TK. Indeed, TK was
able to cleave the C –C bond of 1 to generate the aldehyde 4. A fluorescence
3
2
signal appeared because 4 proved rather unstable and, in the presence of BSA,
underwent a β-elimination to release umbelliferone, a highly fluorescent compound
(Scheme 15.6).
In this way, compound 1 is a substrate for TK, yielding α-hydroxyl and β-
coumarinyl substituted aldehyde. The slow release of fluorescence compared with
the results obtained with transaldolase from an appropriate fluorogenic probe is