Page 387 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 387
17.2 A Generic Strategy for the Synthesis of Sialoconjugate Libraries 363
OH CMP
HO OH HO OH O
CSS
O CO 2 H
AcNH AcNH O CO 2 H
HO OH HO OH
Neu5Ac, 1 CTP PP i
Acceptor
NeuA NeuS Sialidase SiaT
PEP
Pyruvate CMP
HO NHAc OH H
HO O HO CO 2
HO OH AcNH O O Acceptor
ManNAc HO OH
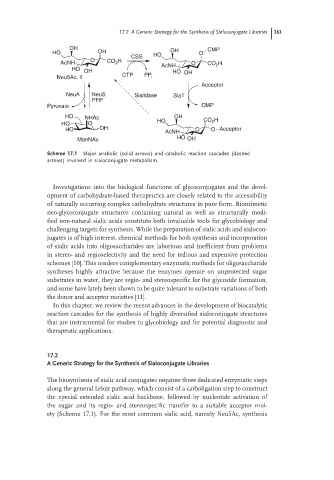
Scheme 17.1 Major anabolic (solid arrows) and catabolic reaction cascades (dashed
arrows) involved in sialoconjugate metabolism.
Investigations into the biological functions of glycoconjugates and the devel-
opment of carbohydrate-based therapeutics are closely related to the accessibility
of naturally occurring complex carbohydrate structures in pure form. Biomimetic
neo-glycoconjugate structures containing natural as well as structurally modi-
fied non-natural sialic acids constitute both invaluable tools for glycobiology and
challenging targets for synthesis. While the preparation of sialic acids and sialocon-
jugates is of high interest, chemical methods for both synthesis and incorporation
of sialic acids into oligosaccharides are laborious and inefficient from problems
in stereo- and regioselectivity and the need for tedious and expensive protection
schemes [10]. This renders complementary enzymatic methods for oligosaccharide
syntheses highly attractive because the enzymes operate on unprotected sugar
substrates in water, they are regio- and stereospecific for the glycoside formation,
and some have lately been shown to be quite tolerant to substrate variations of both
the donor and acceptor moieties [11].
In this chapter, we review the recent advances in the development of biocatalytic
reaction cascades for the synthesis of highly diversified sialoconjugate structures
that are instrumental for studies in glycobiology and for potential diagnostic and
therapeutic applications.
17.2
A Generic Strategy for the Synthesis of Sialoconjugate Libraries
The biosynthesis of sialic acid conjugates requires three dedicated enzymatic steps
along the general Leloir pathway, which consist of a carboligation step to construct
the special extended sialic acid backbone, followed by nucleotide activation of
the sugar and its regio- and stereospecific transfer to a suitable acceptor moi-
ety (Scheme 17.1). For the most common sialic acid, namely Neu5Ac, synthesis