Page 420 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 420
396 18 Methyltransferases in Biocatalysis
− + NH 2
OOC NH 3
N
N
N N
S
H 3 C
O
1
+1
+1 Fe S
Fe S OH OH
S Fe S Fe O O NH 2
S Fe
S Fe N
Fe S Fe S N N
H 2 N N
S
H C
Met 3 O
NH 2
N OH OH
+2 N
Fe S
S Fe N N Met
+ H C
S Fe 2
O
Fe S
3 Fe S +3 NH 2
OH OH
S Fe N
N
S Fe
Fe S N N
NH 2
+ R-H O
N
N
N N OH OH
H 3 C
O
R +
4
OH OH
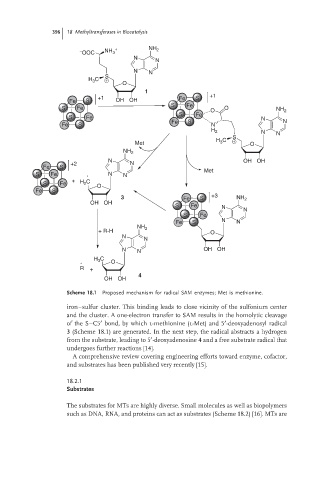
Scheme 18.1 Proposed mechanism for radical SAM enzymes; Met is methionine.
iron–sulfur cluster. This binding leads to close vicinity of the sulfonium center
and the cluster. A one-electron transfer to SAM results in the homolytic cleavage
′ ′
of the S–C5 bond, by which l-methionine (l-Met) and 5 -deoxyadenosyl radical
3 (Scheme 18.1) are generated. In the next step, the radical abstracts a hydrogen
′
from the substrate, leading to 5 -deoxyadenosine 4 and a free substrate radical that
undergoes further reactions [14].
A comprehensive review covering engineering efforts toward enzyme, cofactor,
and substrates has been published very recently [15].
18.2.1
Substrates
The substrates for MTs are highly diverse. Small molecules as well as biopolymers
such as DNA, RNA, and proteins can act as substrates (Scheme 18.2) [16]. MTs are