Page 476 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 476
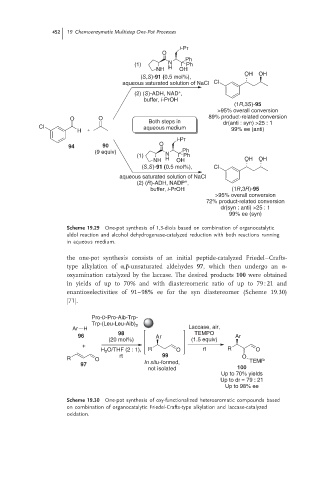
452 19 Chemoenzymatic Multistep One-Pot Processes
i-Pr
O
Ph
(1) N Ph
NH H OH
OH OH
(S,S)-91 (0.5 mol%),
aqueous saturated solution of NaCl Cl
+
(2) (S)-ADH, NAD ,
buffer, i-PrOH
(1R,3S)-95
>95% overall conversion
O O 89% product-related conversion
Both steps in dr(anti : syn) >25 : 1
Cl aqueous medium 99% ee (anti)
H
i-Pr
94 90 O
(9 equiv) Ph
(1) N Ph
NH H OH OH OH
(S,S)-91 (0.5 mol%), Cl
aqueous saturated solution of NaCl
+
(2) (R)-ADH, NADP ,
buffer, i-PrOH (1R,3R)-95
>95% overall conversion
72% product-related conversion
dr(syn : anti) >25 : 1
99% ee (syn)
Scheme 19.29 One-pot synthesis of 1,3-diols based on combination of organocatalytic
aldol reaction and alcohol dehydrogenase-catalyzed reduction with both reactions running
in aqueous medium.
the one-pot synthesis consists of an initial peptide-catalyzed Friedel–Crafts-
type alkylation of α,β-unsaturated aldehydes 97, which then undergo an α-
oxyamination catalyzed by the laccase. The desired products 100 were obtained
in yields of up to 70% and with diastereomeric ratio of up to 79 : 21 and
enantioselectivities of 91–98% ee for the syn diastereomer (Scheme 19.30)
[71].
Pro-D-Pro-Aib-Trp-
Trp-(Leu-Leu-Aib) 2
Ar H Laccase, air,
98 TEMPO
96 Ar Ar
(20 mol%) (1.5 equiv)
+
H 2 O/THF (2 : 1), R O rt R O
rt 99 O
R O TEMP
97 In situ-formed,
not isolated 100
Up to 70% yields
Up to dr = 79 : 21
Up to 98% ee
Scheme 19.30 One-pot synthesis of oxy-functionalized heteroaromatic compounds based
on combination of organocatalytic Friedel-Crafts-type alkylation and laccase-catalyzed
oxidation.