Page 473 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 473
19.4 Combination of Substrate Synthesis and Derivatization Step(s) 449
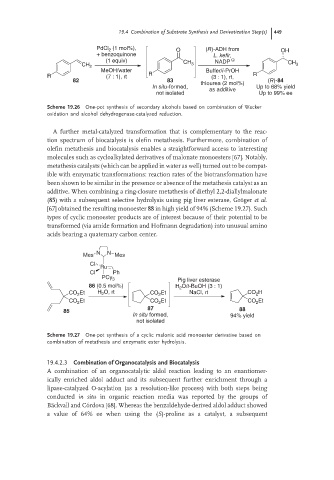
PdCl 2 (1 mol%), O (R)-ADH from OH
+ benzoquinone L. kefir,
(1 equiv) CH NADP
CH 2 3 CH 3
MeOH/water Buffer/i-PrOH
R (7 : 1), rt R (3 : 1), rt, R
82 83 (R)-84
thiourea (2 mol%)
In situ-formed, Up to 68% yield
as additive
not isolated Up to 99% ee
Scheme 19.26 One-pot synthesis of secondary alcohols based on combination of Wacker
oxidation and alcohol dehydrogenase-catalyzed reduction.
A further metal-catalyzed transformation that is complementary to the reac-
tion spectrum of biocatalysis is olefin metathesis. Furthermore, combination of
olefin metathesis and biocatalysis enables a straightforward access to interesting
molecules such as cycloalkylated derivatives of malonate monoesters [67]. Notably,
metathesis catalysts (which can be applied in water as well) turned out to be compat-
ible with enzymatic transformations: reaction rates of the biotransformation have
been shown to be similar in the presence or absence of the metathesis catalyst as an
additive. When combining a ring-closure metathesis of diethyl 2,2-diallylmalonate
(85) with a subsequent selective hydrolysis using pig liver esterase, Gr¨ oger et al.
[67] obtained the resulting monoester 88 in high yield of 94% (Scheme 19.27). Such
types of cyclic monoester products are of interest because of their potential to be
transformed (via amide formation and Hofmann degradation) into unusual amino
acids bearing a quaternary carbon center.
N N
Mes Mes
Cl
Ru
Cl Ph
PCy 3 Pig liver esterase
86 (0.5 mol%) H 2 O/t-BuOH (3 : 1)
CO 2 Et H O, rt CO 2 Et NaCl, rt CO 2 H
2
CO 2 Et CO 2 Et CO 2 Et
87
85 88
In situ formed, 94% yield
not isolated
Scheme 19.27 One-pot synthesis of a cyclic malonic acid monoester derivative based on
combination of metathesis and enzymatic ester hydrolysis.
19.4.2.3 Combination of Organocatalysis and Biocatalysis
A combination of an organocatalytic aldol reaction leading to an enantiomer-
ically enriched aldol adduct and its subsequent further enrichment through a
lipase-catalyzed O-acylation (as a resolution-like process) with both steps being
conducted in situ in organic reaction media was reported by the groups of
B¨ ackvall and C´ ordova [68]. Whereas the benzaldehyde-derived aldol adduct showed
a value of 64% ee when using the (S)-proline as a catalyst, a subsequent