Page 470 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 470
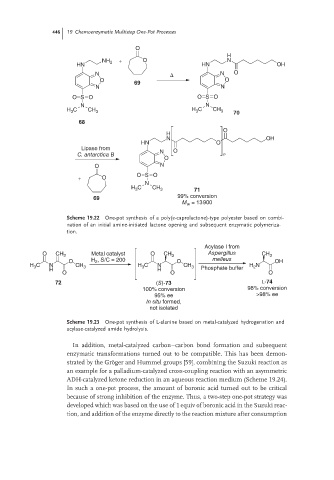
446 19 Chemoenzymatic Multistep One-Pot Processes
O
H
NH 2 + O N
HN HN OH
N Δ N O
O 69 O
N N
O S O O S O
N N
H C CH 3 H C CH 3 70
3
3
68
O
H
N OH
HN O
Lipase from O
C. antarctica B N n
O
O N
O S O
+ O
N
H C CH 3 71
3
69 99% conversion
M = 13900
w
Scheme 19.22 One-pot synthesis of a poly(ε-caprolactone)-type polyester based on combi-
nation of an initial amine-initiated lactone opening and subsequent enzymatic polymeriza-
tion.
Acylase I from
O CH 2 Metal catalyst O CH 3 Aspergillus CH 3
O H 2 , S/C = 200 O melleus OH
H C N CH 3 H C N CH 3 H N
3
2
3
H H Phosphate buffer
O O O
72 (S)-73 L-74
100% conversion 98% conversion
95% ee >98% ee
In situ formed,
not isolated
Scheme 19.23 One-pot synthesis of L-alanine based on metal-catalyzed hydrogenation and
acylase-catalyzed amide hydrolysis.
In addition, metal-catalyzed carbon–carbon bond formation and subsequent
enzymatic transformations turned out to be compatible. This has been demon-
strated by the Gr¨ oger and Hummel groups [59], combining the Suzuki reaction as
an example for a palladium-catalyzed cross-coupling reaction with an asymmetric
ADH-catalyzed ketone reduction in an aqueous reaction medium (Scheme 19.24).
In such a one-pot process, the amount of boronic acid turned out to be critical
because of strong inhibition of the enzyme. Thus, a two-step one-pot strategy was
developed which was based on the use of 1 equiv of boronic acid in the Suzuki reac-
tion, and addition of the enzyme directly to the reaction mixture after consumption