Page 467 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 467
19.4 Combination of Substrate Synthesis and Derivatization Step(s) 443
19.4.2
One-Pot Process with an Initial Chemo Process, Followed by Biocatalysis
19.4.2.1 Combination of Noncatalyzed Organic Reactions and Biocatalysis
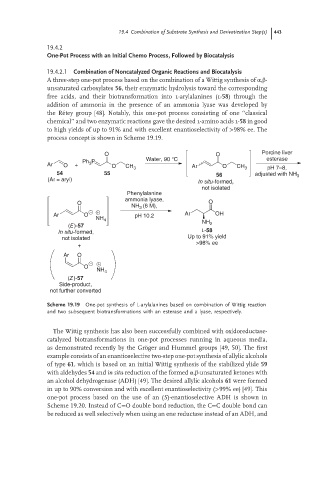
A three-step one-pot process based on the combination of a Wittig synthesis of α,β-
unsaturated carboxylates 56, their enzymatic hydrolysis toward the corresponding
free acids, and their biotransformation into l-arylalanines (l-58) through the
addition of ammonia in the presence of an ammonia lyase was developed by
the R´ etey group [48]. Notably, this one-pot process consisting of one ‘‘classical
chemical’’ and two enzymatic reactions gave the desired l-amino acids l-58 in good
to high yields of up to 91% and with excellent enantioselectivity of >98% ee. The
process concept is shown in Scheme 19.19.
O O Porcine liver
Ph P Water, 90 °C esterase
Ar O + 3 O CH 3 Ar O CH 3 pH 7–8,
54 55 56 adjusted with NH 3
(Ar = aryl) In situ-formed,
not isolated
Phenylalanine
ammonia lyase,
O O
NH 3 (6 M),
Ar O pH 10.2 Ar OH
NH 4
NH 2
(E)-57
In situ-formed, L-58
not isolated Up to 91% yield
>98% ee
+
Ar O
O
NH 4
(Z)-57
Side-product,
not further converted
Scheme 19.19 One-pot synthesis of L-arylalanines based on combination of Wittig reaction
and two subsequent biotransformations with an esterase and a lyase, respectively.
The Wittig synthesis has also been successfully combined with oxidoreductase-
catalyzed biotransformations in one-pot processes running in aqueous media,
as demonstrated recently by the Gr¨ oger and Hummel groups [49, 50]. The first
example consists of an enantioselective two-step one-pot synthesis of allylic alcohols
of type 61, which is based on an initial Wittig synthesis of the stabilized ylide 59
with aldehydes 54 and in situ reduction of the formed α,β-unsaturated ketones with
an alcohol dehydrogenase (ADH) [49]. The desired allylic alcohols 61 were formed
in up to 90% conversion and with excellent enantioselectivity (>99% ee) [49]. This
one-pot process based on the use of an (S)-enantioselective ADH is shown in
Scheme 19.20. Instead of C=O double bond reduction, the C=C double bond can
be reduced as well selectively when using an ene reductase instead of an ADH, and