Page 464 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 464
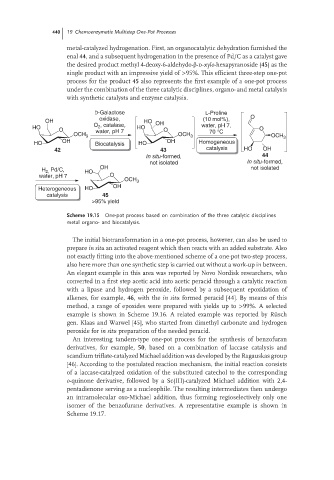
440 19 Chemoenzymatic Multistep One-Pot Processes
metal-catalyzed hydrogenation. First, an organocatalytic dehydration furnished the
enal 44, and a subsequent hydrogenation in the presence of Pd/C as a catalyst gave
the desired product methyl 4-deoxy-6-aldehydo-β-d-xylo-hexapyranoside (45)asthe
single product with an impressive yield of >95%. This efficient three-step one-pot
process for the product 45 also represents the first example of a one-pot process
under the combination of the three catalytic disciplines, organo- and metal catalysis
with synthetic catalysts and enzyme catalysis.
D-Galactose L-Proline
OH oxidase, HO OH (10 mol%), O
HO O 2 , catalase, HO water, pH 7,
O water, pH 7 O 70 °C O
OCH 3 OCH 3 OCH 3
HO OH Biocatalysis HO OH Homogeneous
42 43 catalysis HO OH
In situ-formed, 44
not isolated In situ-formed,
OH not isolated
Pd/C,
H 2, HO
water, pH 7 O
OCH 3
Heterogeneous HO OH
catalysis 45
>95% yield
Scheme 19.15 One-pot process based on combination of the three catalytic disciplines
metal organo- and biocatalysis.
The initial biotransformation in a one-pot process, however, can also be used to
prepare in situ an activated reagent which then reacts with an added substrate. Also
not exactly fitting into the above-mentioned scheme of a one-pot two-step process,
also here more than one synthetic step is carried out without a work-up in between.
An elegant example in this area was reported by Novo Nordisk researchers, who
converted in a first step acetic acid into acetic peracid through a catalytic reaction
with a lipase and hydrogen peroxide, followed by a subsequent epoxidation of
alkenes, for example, 46,withthe in situ formed peracid [44]. By means of this
method, a range of epoxides were prepared with yields up to >99%. A selected
example is shown in Scheme 19.16. A related example was reported by R¨ usch
gen. Klaas and Warwel [45], who started from dimethyl carbonate and hydrogen
peroxide for in situ preparation of the needed peracid.
An interesting tandem-type one-pot process for the synthesis of benzofuran
derivatives, for example, 50, based on a combination of laccase catalysis and
scandium triflate-catalyzed Michael addition was developed by the Ragauskas group
[46]. According to the postulated reaction mechanism, the initial reaction consists
of a laccase-catalyzed oxidation of the substituted catechol to the corresponding
o-quinone derivative, followed by a Sc(III)-catalyzed Michael addition with 2,4-
pentadienone serving as a nucleophile. The resulting intermediates then undergo
an intramolecular oxo-Michael addition, thus forming regioselectively only one
isomer of the benzofurane derivatives. A representative example is shown in
Scheme 19.17.