Page 461 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 461
19.3 Combination of Substrate Isomerization and their Derivatization 437
In spite of the high elegance of this process shown in Scheme 19.11, the need for
the rather expensive pyridoxal 5-phosphate as a catalyst for racemization represents
a drawback. Furthermore, catalyst loading is relatively high with 20 mol%. Since,
however, for in situ formation of the imine the only required function in principle
is an aldehyde group, more simplified aromatic aldehydes have been studied
[34, 35]. The Beller group [34] identified substituted benzaldehyde in particular
when bearing electron-deficient substituents as an efficient substitute for pyridoxal
phosphate, thus leading to a successful DKR of racemic amino esters. For example,
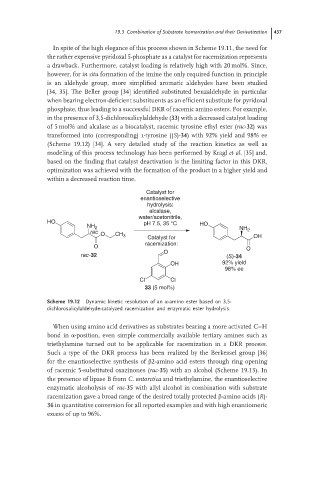
in the presence of 3,5-dichlorosalicylaldehyde (33) with a decreased catalyst loading
of 5 mol% and alcalase as a biocatalyst, racemic tyrosine ethyl ester (rac-32)was
transformed into (corresponding) l-tyrosine ((S)-34) with 92% yield and 98% ee
(Scheme 19.12) [34]. A very detailed study of the reaction kinetics as well as
modeling of this process technology has been performed by Kragl et al. [35] and,
based on the finding that catalyst deactivation is the limiting factor in this DKR,
optimization was achieved with the formation of the product in a higher yield and
within a decreased reaction time.
Catalyst for
enantioselective
hydrolysis:
alcalase,
water/acetonitrile,
HO pH 7.5, 35 °C HO
NH 2
NH 2
rac
O CH 3
Catalyst for OH
racemization:
O O
O
rac-32 (S)-34
OH 92% yield
98% ee
Cl Cl
33 (5 mol%)
Scheme 19.12 Dynamic kinetic resolution of an α-amino ester based on 3,5-
dichlorosalicylaldehyde-catalyzed racemization and enzymatic ester hydrolysis.
When using amino acid derivatives as substrates bearing a more activated C–H
bond in α-position, even simple commercially available tertiary amines such as
triethylamine turned out to be applicable for racemization in a DKR process.
Such a type of the DKR process has been realized by the Berkessel group [36]
for the enantioselective synthesis of β2-amino acid esters through ring opening
of racemic 5-substituted oxazinones (rac-35) with an alcohol (Scheme 19.13). In
the presence of lipase B from C. antarctica and triethylamine, the enantioselective
enzymatic alcoholysis of rac-35 with allyl alcohol in combination with substrate
racemization gave a broad range of the desired totally protected β-amino acids (R)-
36 in quantitative conversion for all reported examples and with high enantiomeric
excess of up to 96%.