Page 456 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 456
432 19 Chemoenzymatic Multistep One-Pot Processes
reaction was then successfully combined with a lipase-catalyzed resolution toward
a DKR process for a broad range of secondary alcohols, which were typically
◦
transformed at elevated temperature (70 C) into their esters of type (R)-15 in high
yields of 60–92% and with excellent enantioselectivities of upto >99% ee [13, 14].
As an acyl donor, p-chlorophenyl acetate was used (Scheme 19.5).
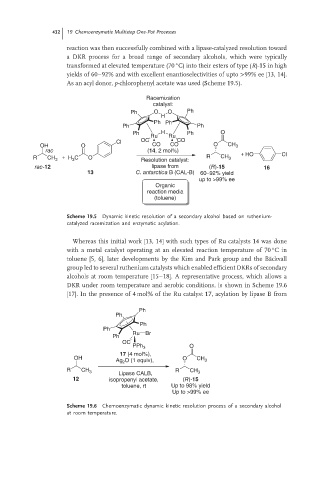
Racemization
catalyst:
Ph O O Ph
H
Ph Ph
Ph Ph
Ph H Ph O
Ru Ru
Cl OC CO
OH O CO CO O CH 3
rac (14, 2 mol%) + HO
R CH 3 + H 3 C O Resolution catalyst: R CH 3 Cl
rac-12 lipase from (R)-15 16
13 C. antarctica B (CAL-B) 60–92% yield
up to >99% ee
Organic
reaction media
(toluene)
Scheme 19.5 Dynamic kinetic resolution of a secondary alcohol based on ruthenium-
catalyzed racemization and enzymatic acylation.
Whereas this initial work [13, 14] with such types of Ru catalysts 14 was done
◦
with a metal catalyst operating at an elevated reaction temperature of 70 Cin
toluene [5, 6], later developments by the Kim and Park group and the B¨ ackvall
group led to several ruthenium catalysts which enabled efficient DKRs of secondary
alcohols at room temperature [15–18]. A representative process, which allows a
DKR under room temperature and aerobic conditions, is shown in Scheme 19.6
[17]. In the presence of 4 mol% of the Ru catalyst 17, acylation by lipase B from
Ph
Ph
Ph
Ph
Ru Br
Ph
OC
PPh 3 O
17 (4 mol%),
OH Ag 2 O (1 equiv), O CH 3
R CH 3 Lipase CALB, R CH 3
12 isopropenyl acetate, (R)-15
toluene, rt Up to 98% yield
Up to >99% ee
Scheme 19.6 Chemoenzymatic dynamic kinetic resolution process of a secondary alcohol
at room temperature.