Page 457 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 457
19.3 Combination of Substrate Isomerization and their Derivatization 433
Candida antarctica (CAL-B) led to the formation of the esters (R)-15 in up to 98%
yield and with upto 99% ee.
This DKR principle, based on metal-catalyzed racemization via reversible redox
reactions with alcohols and lipase-catalyzed O-acylation as the enantioselective
step, has been applied to a wide range of alcohols [5, 6]. In the following,
selected examples will be given. Besides a range of 1-arylethan-1-ols and 1-
heteroarylethan-1-ols [5, 6], also racemic secondary alcohols bearing two sterically
demanding substituents were transformed successfully into their esters with both
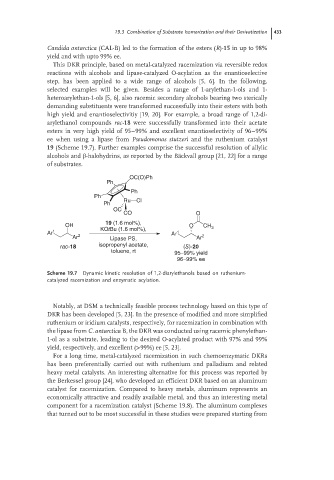
high yield and enantioselectivitiy [19, 20]. For example, a broad range of 1,2-di-
arylethanol compounds rac-18 were successfully transformed into their acetate
esters in very high yield of 95–99% and excellent enantioselectivity of 96–99%
ee when using a lipase from Pseudomonas stutzeri and the ruthenium catalyst
19 (Scheme 19.7). Further examples comprise the successful resolution of allylic
alcohols and β-halohydrins, as reported by the B¨ ackvall group [21, 22] for a range
of substrates.
OC(O)Ph
Ph
Ph
Ph
Ru Cl
Ph
OC
CO O
19 (1.6 mol%),
OH O CH 3
KOtBu (1.6 mol%),
Ar 1 Ar 1
Ar 2 Lipase PS, Ar 2
rac-18 isopropenyl acetate, (S)-20
toluene, rt 95–99% yield
96–99% ee
Scheme 19.7 Dynamic kinetic resolution of 1,2-diarylethanols based on ruthenium-
catalyzed racemization and enzymatic acylation.
Notably, at DSM a technically feasible process technology based on this type of
DKR has been developed [5, 23]. In the presence of modified and more simplified
ruthenium or iridium catalysts, respectively, for racemization in combination with
the lipase from C. antarctica B, the DKR was conducted using racemic phenylethan-
1-ol as a substrate, leading to the desired O-acylated product with 97% and 99%
yield, respectively, and excellent (>99%) ee [5, 23].
For a long time, metal-catalyzed racemization in such chemoenzymatic DKRs
has been preferentially carried out with ruthenium and palladium and related
heavy metal catalysts. An interesting alternative for this process was reported by
the Berkessel group [24], who developed an efficient DKR based on an aluminum
catalyst for racemization. Compared to heavy metals, aluminum represents an
economically attractive and readily available metal, and thus an interesting metal
component for a racemization catalyst (Scheme 19.8). The aluminum complexes
that turned out to be most successful in these studies were prepared starting from