Page 462 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 462
438 19 Chemoenzymatic Multistep One-Pot Processes
O
Lipase from
R C. antarctica B O O
O
+ allyl alcohol, Ph N O
N Ph toluene, rt H R
(R)-35 (R)-36
quantitative conversion
NEt 3
(6–50 mol%) 76–96% ee
O
R
O
N Ph
(S)-35
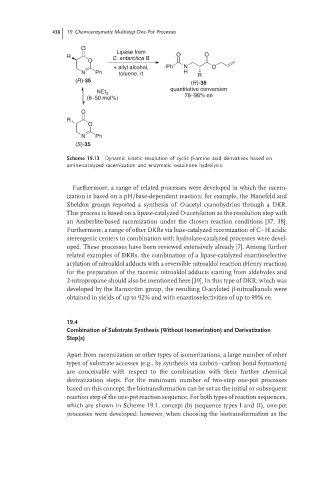
Scheme 19.13 Dynamic kinetic resolution of cyclic β-amino acid derivatives based on
amine-catalyzed racemization and enzymatic oxazinone hydrolysis.
Furthermore, a range of related processes were developed in which the racem-
ization is based on a pH/base-dependent reaction: for example, the Hanefeld and
Sheldon groups reported a synthesis of O-acetyl cyanohydrins through a DKR.
This process is based on a lipase-catalyzed O-acetylation as the resolution step with
an Amberlite-based racemization under the chosen reaction conditions [37, 38].
Furthermore, a range of other DKRs via base-catalyzed racemization of C–H acidic
stereogenic centers in combination with hydrolase-catalyzed processes were devel-
oped. These processes have been reviewed extensively already [7]. Among further
related examples of DKRs, the combination of a lipase-catalyzed enantioselective
acylation of nitroaldol adducts with a reversible nitroaldol reaction (Henry reaction)
for the preparation of the racemic nitroaldol adducts starting from aldehydes and
2-nitropropane should also be mentioned here [39]. In this type of DKR, which was
developed by the Ramstr¨ om group, the resulting O-acylated β-nitroalkanols were
obtained in yields of up to 92% and with enantioselectivities of up to 99% ee.
19.4
Combination of Substrate Synthesis (Without Isomerization) and Derivatization
Step(s)
Apart from racemization or other types of isomerizations, a large number of other
types of substrate accesses (e.g., by synthesis via carbon–carbon bond formation)
are conceivable with respect to the combination with their further chemical
derivatization steps. For the minimum number of two-step one-pot processes
based on this concept, the biotransformation can be set as the initial or subsequent
reaction step of the one-pot reaction sequence. For both types of reaction sequences,
which are shown in Scheme 19.1, concept (b) (sequence types I and II), one-pot
processes were developed: however, when choosing the biotransformation as the