Page 465 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 465
19.4 Combination of Substrate Synthesis and Derivatization Step(s) 441
H 2 O H 2 O 2
Lipase from
C. antarctica B
O O
O H
H C O H H C O
3
3
O
n
46 n
47
n = 0: >99% yield
n = 1: 98% yield
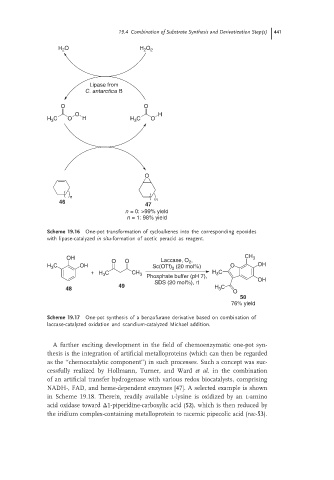
Scheme 19.16 One-pot transformation of cycloalkenes into the corresponding epoxides
with lipase-catalyzed in situ-formation of acetic peracid as reagent.
OH CH 3
O O Laccase, O 2 ,
H C OH Sc(OTf) 3 (20 mol%) O OH
3
+ H C CH 3 Phosphate buffer (pH 7), H C
3
3
SDS (20 mol%), rt OH
49
48 H 3 C
O
50
76% yield
Scheme 19.17 One-pot synthesis of a benzofurane derivative based on combination of
laccase-catalyzed oxidation and scandium-catalyzed Michael addition.
A further exciting development in the field of chemoenzymatic one-pot syn-
thesis is the integration of artificial metalloproteins (which can then be regarded
as the ‘‘chemocatalytic component’’) in such processes. Such a concept was suc-
cessfully realized by Hollmann, Turner, and Ward et al. in the combination
of an artificial transfer hydrogenase with various redox biocatalysts, comprising
NADH-, FAD, and heme-dependent enzymes [47]. A selected example is shown
in Scheme 19.18. Therein, readily available l-lysine is oxidized by an l-amino
acid oxidase toward Δ1-piperidine-carboxylic acid (52), which is then reduced by
the iridium complex-containing metalloprotein to racemic pipecolic acid (rac-53).