Page 468 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 468
444 19 Chemoenzymatic Multistep One-Pot Processes
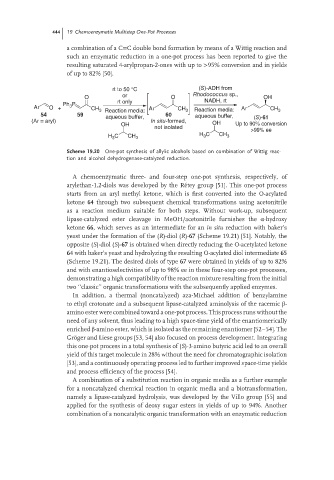
a combination of a C=C double bond formation by means of a Wittig reaction and
such an enzymatic reduction in a one-pot process has been reported to give the
resulting saturated 4-arylpropan-2-ones with up to >95% conversion and in yields
of up to 82% [50].
rt to 50 °C (S)-ADH from
O or O Rhodococcus sp., OH
rt only NADH, rt
Ph 3 P
Ar O + CH 3 Reaction media: Ar CH 3 Reaction media: Ar CH 3
54 59 aqueous buffer, 60 aqueous buffer, (S)-61
(Ar = aryl) In situ-formed,
OH OH Up to 90% conversion
not isolated >99% ee
H 3 C CH 3 H C CH 3
3
Scheme 19.20 One-pot synthesis of allylic alcohols based on combination of Wittig reac-
tion and alcohol dehydrogenase-catalyzed reduction.
A chemoenzymatic three- and four-step one-pot synthesis, respectively, of
arylethan-1,2-diols was developed by the R´ etey group [51]. This one-pot process
starts from an aryl methyl ketone, which is first converted into the O-acylated
ketone 64 through two subsequent chemical transformations using acetonitrile
as a reaction medium suitable for both steps. Without work-up, subsequent
lipase-catalyzed ester cleavage in MeOH/acetonitrile furnishes the α-hydroxy
ketone 66, which serves as an intermediate for an in situ reduction with baker’s
yeast under the formation of the (R)-diol (R)-67 (Scheme 19.21) [51]. Notably, the
opposite (S)-diol (S)-67 is obtained when directly reducing the O-acetylated ketone
64 with baker’s yeast and hydrolyzing the resulting O-acylated diol intermediate 65
(Scheme 19.21). The desired diols of type 67 were obtained in yields of up to 82%
and with enantioselectivities of up to 98% ee in these four-step one-pot processes,
demonstrating a high compatibility of the reaction mixture resulting from the initial
two ‘‘classic’’ organic transformations with the subsequently applied enzymes.
In addition, a thermal (noncatalyzed) aza-Michael addition of benzylamine
to ethyl crotonate and a subsequent lipase-catalyzed aminolysis of the racemic β-
amino ester were combined toward a one-pot process. This process runs without the
need of any solvent, thus leading to a high space-time yield of the enantiomerically
enriched β-amino ester, which is isolated as the remaining enantiomer [52–54]. The
Gr¨ oger and Liese groups [53, 54] also focused on process development. Integrating
this one-pot process in a total synthesis of (S)-3-amino butyric acid led to an overall
yield of this target molecule in 28% without the need for chromatographic isolation
[53], and a continuously operating process led to further improved space-time yields
and process efficiency of the process [54].
A combination of a substitution reaction in organic media as a further example
for a noncatalyzed chemical reaction in organic media and a biotransformation,
namely a lipase-catalyzed hydrolysis, was developed by the Villo group [55] and
applied for the synthesis of deoxy sugar esters in yields of up to 94%. Another
combination of a noncatalytic organic transformation with an enzymatic reduction