Page 471 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 471
19.4 Combination of Substrate Synthesis and Derivatization Step(s) 447
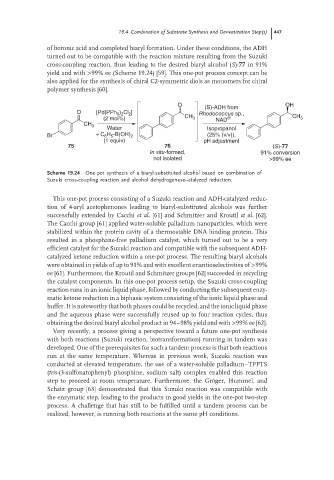
of boronic acid and completed biaryl formation. Under these conditions, the ADH
turned out to be compatible with the reaction mixture resulting from the Suzuki
cross-coupling reaction, thus leading to the desired biaryl alcohol (S)-77 in 91%
yield and with >99% ee (Scheme 19.24) [59]. This one-pot process concept can be
also applied for the synthesis of chiral C2-symmetric diols as monomers for chiral
polymer synthesis [60].
O OH
(S)-ADH from
O [Pd(PPh 3 ) 2 Cl 2 ] Rhodococcus sp.,
(2 mol%) CH 3 NAD CH 3
CH 3
Water Isopropanol
Br + C 6 H 5 -B(OH) 2 (25% (v/v)),
(1 equiv) pH adjustment
75 76 (S)-77
In situ-formed, 91% conversion
not isolated >99% ee
Scheme 19.24 One-pot synthesis of a biaryl-substituted alcohol based on combination of
Suzuki cross-coupling reaction and alcohol dehydrogenase--atalyzed reduction.
This one-pot process consisting of a Suzuki reaction and ADH-catalyzed reduc-
tion of 4-aryl acetophenones leading to biaryl-substituted alcohols was further
successfully extended by Cacchi et al. [61] and Schmitzer and Kroutil et al. [62].
The Cacchi group [61] applied water-soluble palladium nanoparticles, which were
stabilized within the protein cavity of a thermostable DNA binding protein. This
resulted in a phosphane-free palladium catalyst, which turned out to be a very
efficient catalyst for the Suzuki reaction and compatible with the subsequent ADH-
catalyzed ketone reduction within a one-pot process. The resulting biaryl alcohols
were obtained in yields of up to 91% and with excellent enantioselectivities of >99%
ee [61]. Furthermore, the Kroutil and Schmitzer groups [62] succeeded in recycling
the catalyst components. In this one-pot process setup, the Suzuki cross-coupling
reaction runs in an ionic liquid phase, followed by conducting the subsequent enzy-
matic ketone reduction in a biphasic system consisting of the ionic liquid phase and
buffer. It is noteworthy that both phases could be recycled, and the ionic liquid phase
and the aqueous phase were successfully reused up to four reaction cycles, thus
obtaining the desired biaryl alcohol product in 94–98% yield and with >99% ee [62].
Very recently, a process giving a perspective toward a future one-pot synthesis
with both reactions (Suzuki reaction, biotransformation) running in tandem was
developed. One of the prerequisites for such a tandem process is that both reactions
run at the same temperature. Whereas in previous work, Suzuki reaction was
conducted at elevated temperature, the use of a water-soluble palladium–TPPTS
(tris-(3-sulfonatophenyl) phosphine, sodium salt) complex enabled this reaction
step to proceed at room temperature. Furthermore, the Gr¨ oger, Hummel, and
Schatz group [63] demonstrated that this Suzuki reaction was compatible with
the enzymatic step, leading to the products in good yields in the one-pot two-step
process. A challenge that has still to be fulfilled until a tandem process can be
realized, however, is running both reactions at the same pH conditions.