Page 469 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 469
19.4 Combination of Substrate Synthesis and Derivatization Step(s) 445
OH OH
Hydrolase
O CH OH
R 3 R
65 O
(S)-67
Sodium Baker’s yeast
Polymer-bound acetate,
O pyridinium O 18C6-crown O
tribromide ether
R CH 3 Acetonitrile R Br Acetonitrile R O CH 3
62 63 64 O
(R = C H , R = Cl-C H ) Lipase CALB,
6 4
6 5
methanol,
acetonitrile
O Baker’s yeast OH
OH OH
R R
66
(R)-67
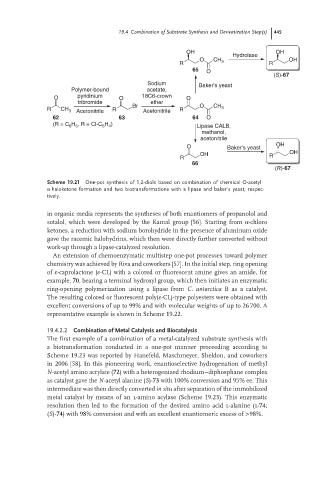
Scheme 19.21 One-pot synthesis of 1,2-diols based on combination of chemical O-acetyl
α-haloketone formation and two biotransformations with a lipase and baker’s yeast, respec-
tively.
in organic media represents the syntheses of both enantiomers of propanolol and
sotalol, which were developed by the Kamal group [56]. Starting from α-chloro
ketones, a reduction with sodium borohydride in the presence of aluminum oxide
gave the racemic halohydrins, which then were directly further converted without
work-up through a lipase-catalyzed resolution.
An extension of chemoenzymatic multistep one-pot processes toward polymer
chemistry was achieved by Riva and coworkers [57]. In the initial step, ring opening
of ε-caprolactone (ε-CL) with a colored or fluorescent amine gives an amide, for
example, 70, bearing a terminal hydroxyl group, which then initiates an enzymatic
ring-opening polymerization using a lipase from C. antarctica Basacatalyst.
The resulting colored or fluorescent poly(ε-CL)-type polyesters were obtained with
excellent conversions of up to 99% and with molecular weights of up to 26 700. A
representative example is shown in Scheme 19.22.
19.4.2.2 Combination of Metal Catalysis and Biocatalysis
The first example of a combination of a metal-catalyzed substrate synthesis with
a biotransformation conducted in a one-pot manner proceeding according to
Scheme 19.23 was reported by Hanefeld, Maschmeyer, Sheldon, and coworkers
in 2006 [58]. In this pioneering work, enantioselective hydrogenation of methyl
N-acetyl amino acrylate (72) with a heterogenized rhodium–diphosphane complex
as catalyst gave the N-acetyl alanine (S)-73 with 100% conversion and 95% ee. This
intermediate was then directly converted in situ after separation of the immobilized
metal catalyst by means of an l-amino acylase (Scheme 19.23). This enzymatic
resolution then led to the formation of the desired amino acid l-alanine (l-74;
(S)-74) with 98% conversion and with an excellent enantiomeric excess of >98%.