Page 454 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 454
430 19 Chemoenzymatic Multistep One-Pot Processes
with glutaraldehyde) and Pt/C (5%) as heterogeneous catalyst in combination
with 20 atm of hydrogen, composition of the reaction mixture showed an amount
of 46% each for the products d-mannitol and d-glucitol, while d-glucose (5%)
and d-fructose (3%) were found as minor components [8]. Later work addressed
process development by varying both immobilized d-glucose isomerase and the
metal catalyst component, respectively [9, 10]. Stewart and Ruddlesden [9] applied a
ruthenium-loaded zeolite as a catalyst in combination with a d-glucose isomerase,
thus obtaining d-mannitol in 29% yield when starting from d-glucose. The van
Bekkum group [10] developed a further improved process based on a d-glucose
isomerase immobilized on silica in combination with a copper-on-silica catalyst. By
means of these catalytic components, this isomerization–hydrogenation process
runs efficiently with the formation of d-mannitol in high yields of 62–66%. As
substrates, the use of a 1 : 1 d-glucose/d-fructose mixture as well as d-glucose alone
was reported to be suitable.
After this pioneering work by van Bekkum et al., further breakthroughs were
achieved by several groups in the 1990s in the field of combining a chemocatalytic
racemization (chosen as a specific type of isomerization) and a hydrolase-catalyzed
process toward DKRs. It is noteworthy that a wide variety of different strategies
based on different types of chemo- and biocatalysts as well as different reaction
media (organic and aqueous solvents) have been realized, all fulfilling the criteria
of a DKR.
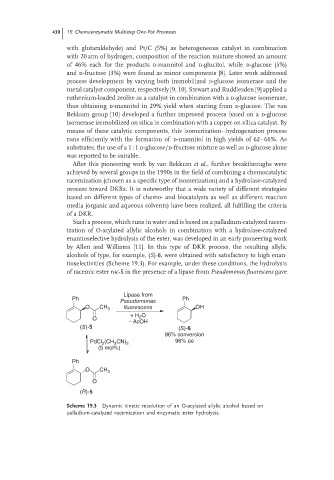
Such a process, which runs in water and is based on a palladium-catalyzed racem-
ization of O-acylated allylic alcohols in combination with a hydrolase-catalyzed
enantioselective hydrolysis of the ester, was developed in an early pioneering work
by Allen and Williams [11]. In this type of DKR process, the resulting allylic
alcohols of type, for example, (S)-6, were obtained with satisfactory to high enan-
tioselectivities (Scheme 19.3). For example, under these conditions, the hydrolysis
of racemic ester rac-5 in the presence of a lipase from Pseudomonas fluorescens gave
Lipase from
Ph Ph
Pseudomonas
O CH 3 fluorescens OH
O
+ H 2
O
− AcOH
(S)-5 (S)-6
96% conversion
PdCl (CH CN) 2 96% ee
3
2
(5 mol%)
Ph
O CH 3
O
(R)-5
Scheme 19.3 Dynamic kinetic resolution of an O-acylated allylic alcohol based on
palladium-catalyzed racemization and enzymatic ester hydrolysis.