Page 52 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 52
28 2 New Trends in the In Situ Enzymatic Recycling of NAD(P)(H) Cofactors
However, recent studies have shown that especially with [NiFe] hydrogenases
somekindofO tolerance can be observed, where in most cases O behaves
2 2
as a reversible inhibitor rather than an agent that effectively causes irreversible
inactivation [34].
One of the first hydrogenases investigated for the regeneration of reduced
nicotinamide cofactors is the cytoplasmic [NiFe] hydrogenase from Ralstonia
eutropha (formerly Alcaligenes eutrophus) [35]. Although in the earlier studies,
molecular oxygen was removed from the reaction mixture (both by applying
vacuum and by enzymatic treatment with glucose oxidase in the presence of
glucose) to avoid hydrogenase inactivation, it is now generally assumed that this
hydrogenase is sufficiently O tolerant. A deeper biochemical characterization of
2
the R. eutropha hydrogenase has recently revealed its quite complicated modular
structure, comprising six subunits organized in two main catalytic moieties,
that is, the real hydrogenase (HoxHY) and the diaphorase responsible for the
+
NAD /NADH cycling (HoxFU) [36]. It has also been shown that these two moieties
can be independently expressed and purified, both retaining their respective
catalytic activities. Therefore, in a recent communication, it has been suggested
to couple the individually expressed diaphorase with more robust hydrogenases,
because HoxHY is less stable than HoxFU for the possible loss of its flavin
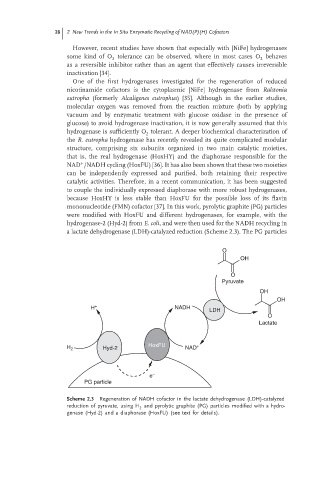
mononucleotide (FMN) cofactor [37]. In this work, pyrolytic graphite (PG) particles
were modified with HoxFU and different hydrogenases, for example, with the
hydrogenase-2 (Hyd-2) from E. coli, and were then used for the NADH recycling in
a lactate dehydrogenase (LDH)-catalyzed reduction (Scheme 2.3). The PG particles
O
OH
O
Pyruvate
OH
OH
H + NADH LDH
O
Lactate
H 2 Hyd-2 HoxFU NAD +
−
e
PG particle
Scheme 2.3 Regeneration of NADH cofactor in the lactate dehydrogenase (LDH)-catalyzed
reduction of pyruvate, using H and pyrolytic graphite (PG) particles modified with a hydro-
2
genase (Hyd-2) and a diaphorase (HoxFU) (see text for details).