Page 55 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 55
2.2 Advances in Cofactor Regeneration 31
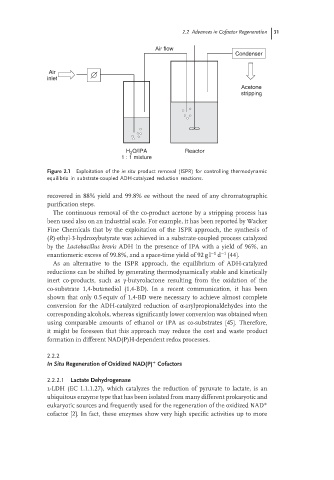
Air flow
Condenser
Air
inlet
Acetone
stripping
H O/IPA Reactor
2
1 : 1 mixture
Figure 2.1 Exploitation of the in situ product removal (ISPR) for controlling thermodynamic
equilibria in substrate-coupled ADH-catalyzed reduction reactions.
recovered in 88% yield and 99.8% ee without the need of any chromatographic
purification steps.
The continuous removal of the co-product acetone by a stripping process has
been used also on an industrial scale. For example, it has been reported by Wacker
Fine Chemicals that by the exploitation of the ISPR approach, the synthesis of
(R)-ethyl-3-hydroxybutyrate was achieved in a substrate-coupled process catalyzed
by the Lactobacillus brevis ADH in the presence of IPA with a yield of 96%, an
enantiomeric excess of 99.8%, and a space-time yield of 92 g l −1 d −1 [44].
As an alternative to the ISPR approach, the equilibrium of ADH-catalyzed
reductions can be shifted by generating thermodynamically stable and kinetically
inert co-products, such as γ-butyrolactone resulting from the oxidation of the
co-substrate 1,4-butanediol (1,4-BD). In a recent communication, it has been
shown that only 0.5 equiv of 1,4-BD were necessary to achieve almost complete
conversion for the ADH-catalyzed reduction of α-arylpropionaldehydes into the
corresponding alcohols, whereas significantly lower conversion was obtained when
using comparable amounts of ethanol or IPA as co-substrates [45]. Therefore,
it might be foreseen that this approach may reduce the cost and waste product
formation in different NAD(P)H-dependent redox processes.
2.2.2
+
In Situ Regeneration of Oxidized NAD(P) Cofactors
2.2.2.1 Lactate Dehydrogenase
l-LDH (EC 1.1.1.27), which catalyzes the reduction of pyruvate to lactate, is an
ubiquitous enzyme type that has been isolated from many different prokaryotic and
+
eukaryotic sources and frequently used for the regeneration of the oxidized NAD
cofactor [2]. In fact, these enzymes show very high specific activities up to more