Page 59 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 59
2.2 Advances in Cofactor Regeneration 35
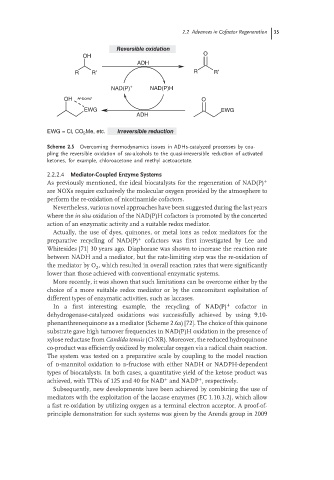
Reversible oxidation
OH O
ADH
R R′ R R′
NAD(P) + NAD(P)H
OH H-bond O
EWG EWG
ADH
EWG = Cl, CO 2 Me, etc. Irreversible reduction
Scheme 2.5 Overcoming thermodynamics issues in ADHs-catalyzed processes by cou-
pling the reversible oxidation of sec-alcohols to the quasi-irreversible reduction of activated
ketones, for example, chloroacetone and methyl acetoacetate.
2.2.2.4 Mediator-Coupled Enzyme Systems
As previously mentioned, the ideal biocatalysts for the regeneration of NAD(P) +
are NOXs require exclusively the molecular oxygen provided by the atmosphere to
perform the re-oxidation of nicotinamide cofactors.
Nevertheless, various novel approaches have been suggested during the last years
where the in situ oxidation of the NAD(P)H cofactors is promoted by the concerted
action of an enzymatic activity and a suitable redox mediator.
Actually, the use of dyes, quinones, or metal ions as redox mediators for the
preparative recycling of NAD(P) + cofactors was first investigated by Lee and
Whitesides [71] 30 years ago. Diaphorase was shown to increase the reaction rate
between NADH and a mediator, but the rate-limiting step was the re-oxidation of
the mediator by O , which resulted in overall reaction rates that were significantly
2
lower than those achieved with conventional enzymatic systems.
More recently, it was shown that such limitations can be overcome either by the
choice of a more suitable redox mediator or by the concomitant exploitation of
different types of enzymatic activities, such as laccases.
In a first interesting example, the recycling of NAD(P) + cofactor in
dehydrogenase-catalyzed oxidations was successfully achieved by using 9,10-
phenanthrenequinone as a mediator (Scheme 2.6a) [72]. The choice of this quinone
substrate gave high turnover frequencies in NAD(P)H oxidation in the presence of
xylose reductase from Candida tenuis (Ct-XR). Moreover, the reduced hydroquinone
co-product was efficiently oxidized by molecular oxygen via a radical chain reaction.
The system was tested on a preparative scale by coupling to the model reaction
of d-mannitol oxidation to d-fructose with either NADH or NADPH-dependent
types of biocatalysts. In both cases, a quantitative yield of the ketose product was
+
+
achieved, with TTNs of 125 and 40 for NAD and NADP , respectively.
Subsequently, new developments have been achieved by combining the use of
mediators with the exploitation of the laccase enzymes (EC 1.10.3.2), which allow
a fast re-oxidation by utilizing oxygen as a terminal electron acceptor. A proof-of-
principle demonstration for such systems was given by the Arends group in 2009