Page 54 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 54
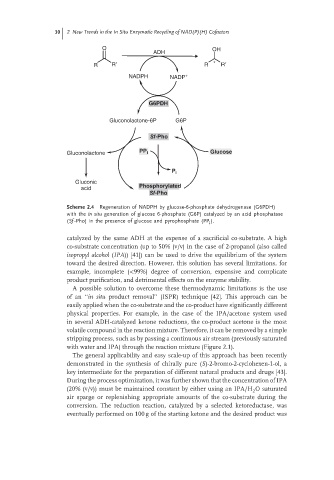
30 2 New Trends in the In Situ Enzymatic Recycling of NAD(P)(H) Cofactors
O OH
ADH
R R′ R * R′
NADPH NADP +
G6PDH
Gluconolactone-6P G6P
Sf-Pho
Gluconolactone PP i Glucose
P i
Gluconic
acid Phosphorylated
Sf-Pho
Scheme 2.4 Regeneration of NADPH by glucose-6-phosphate dehydrogenase (G6PDH)
with the in situ generation of glucose 6-phosphate (G6P) catalyzed by an acid phosphatase
(Sf -Pho) in the presence of glucose and pyrophosphate (PP ).
i
catalyzed by the same ADH at the expense of a sacrificial co-substrate. A high
co-substrate concentration (up to 50% (v/v) in the case of 2-propanol (also called
isopropyl alcohol (IPA)) [41]) can be used to drive the equilibrium of the system
toward the desired direction. However, this solution has several limitations, for
example, incomplete (<99%) degree of conversion, expensive and complicate
product purification, and detrimental effects on the enzyme stability.
A possible solution to overcome these thermodynamic limitations is the use
of an ‘‘in situ product removal’’ (ISPR) technique [42]. This approach can be
easily applied when the co-substrate and the co-product have significantly different
physical properties. For example, in the case of the IPA/acetone system used
in several ADH-catalyzed ketone reductions, the co-product acetone is the most
volatile compound in the reaction mixture. Therefore, it can be removed by a simple
stripping process, such as by passing a continuous air stream (previously saturated
with water and IPA) through the reaction mixture (Figure 2.1).
The general applicability and easy scale-up of this approach has been recently
demonstrated in the synthesis of chirally pure (S)-2-bromo-2-cyclohexen-1-ol, a
key intermediate for the preparation of different natural products and drugs [43].
During the process optimization, it was further shown that the concentration of IPA
(20% (v/v)) must be maintained constant by either using an IPA/H O saturated
2
air sparge or replenishing appropriate amounts of the co-substrate during the
conversion. The reduction reaction, catalyzed by a selected ketoreductase, was
eventually performed on 100 g of the starting ketone and the desired product was