Page 57 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 57
2.2 Advances in Cofactor Regeneration 33
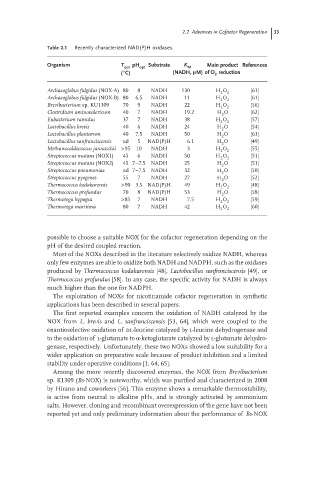
Table 2.1 Recently characterized NAD(P)H oxidases.
Organism T opt pH opt Substrate K M Main product References
◦
( C) (NADH, M) of O reduction
2
Archaeoglobus fulgidus (NOX-A) 80 8 NADH 130 H O 2 [61]
2
Archaeoglobus fulgidus (NOX-B) 80 6.5 NADH 11 H O 2 [61]
2
Brevibacterium sp. KU1309 70 9 NADH 22 H O [56]
2 2
Clostridium aminovalericum 40 7 NADH 19.2 H O [62]
2
Eubacterium ramulus 37 7 NADH 38 H O [57]
2 2
Lactobacillus brevis 40 6 NADH 24 H O [54]
2
Lactobacillus plantarum 40 7.5 NADH 50 H O [63]
2
Lactobacillus sanfranciscensis nd 5 NAD(P)H 6.1 H O [49]
2
Methanocaldococcus jannaschii >95 10 NADH 3 H O [55]
2 2
Streptococcus mutans (NOX1) 45 6 NADH 50 H O 2 [51]
2
Streptococcus mutans (NOX2) 45 7–7.5 NADH 25 H O [51]
2
Streptococcus pneumoniae nd 7–7.5 NADH 32 H O [50]
2
Streptococcus pyogenes 55 7 NADH 27 H O [52]
2
Thermococcus kodakarensis >90 3.5 NAD(P)H 49 H O 2 [48]
2
Thermococcus profundus 70 8 NAD(P)H 53 H O [58]
2
Thermotoga hypogea >85 7 NADH 7.5 H O [59]
2 2
Thermotoga maritima 80 7 NADH 42 H O 2 [60]
2
possible to choose a suitable NOX for the cofactor regeneration depending on the
pH of the desired coupled reaction.
Most of the NOXs described in the literature selectively oxidize NADH, whereas
only few enzymes are able to oxidize both NADH and NADPH, such as the oxidases
produced by Thermococcus kodakarensis [48], Lactobacillus sanfranciscensis [49], or
Thermococcus profundus [58]. In any case, the specific activity for NADH is always
much higher than the one for NADPH.
The exploitation of NOXs for nicotinamide cofactor regeneration in synthetic
applications has been described in several papers.
The first reported examples concern the oxidation of NADH catalyzed by the
NOX from L. brevis and L. sanfranciscensis [53, 64], which were coupled to the
enantioselective oxidation of dl-leucine catalyzed by l-leucine dehydrogenase and
to the oxidation of l-glutamate to α-ketoglutarate catalyzed by l-glutamate dehydro-
genase, respectively. Unfortunately, these two NOXs showed a low suitability for a
wider application on preparative scale because of product inhibition and a limited
stability under operative conditions [1, 64, 65].
Among the more recently discovered enzymes, the NOX from Brevibacterium
sp. K1309 (Bs-NOX) is noteworthy, which was purified and characterized in 2008
by Hirano and coworkers [56]. This enzyme shows a remarkable thermostability,
is active from neutral to alkaline pHs, and is strongly activated by ammonium
salts. However, cloning and recombinant overexpression of the gene have not been
reported yet and only preliminary information about the performance of Bs-NOX