Page 92 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 92
68 4 Biocatalytic Redox Cascades Involving -Transaminases
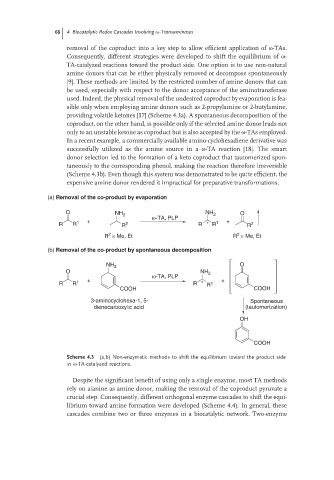
removal of the coproduct into a key step to allow efficient application of ω-TAs.
Consequently, different strategies were developed to shift the equilibrium of ω-
TA-catalyzed reactions toward the product side. One option is to use non-natural
amine donors that can be either physically removed or decompose spontaneously
[9]. These methods are limited by the restricted number of amine donors that can
be used, especially with respect to the donor acceptance of the aminotransferase
used. Indeed, the physical removal of the undesired coproduct by evaporation is fea-
sible only when employing amine donors such as 2-propylamine or 2-butylamine,
providing volatile ketones [17] (Scheme 4.3a). A spontaneous decomposition of the
coproduct, on the other hand, is possible only if the selected amine donor leads not
only to an unstable ketone as coproduct but is also accepted by the ω-TAs employed.
In a recent example, a commercially available amino-cyclohexadiene derivative was
successfully utilized as the amine source in a ω-TA reaction [18]. The smart
donor selection led to the formation of a keto coproduct that tautomerized spon-
taneously to the corresponding phenol, making the reaction therefore irreversible
(Scheme 4.3b). Even though this system was demonstrated to be quite efficient, the
expensive amine donor rendered it impractical for preparative transformations.
(a) Removal of the co-product by evaporation
O NH 2 NH 2 O
ω-TA, PLP
R R 1 + R 2 R * R 1 + R 2
2
2
R = Me, Et R = Me, Et
(b) Removal of the co-product by spontaneous decomposition
NH 2 O
O NH 2
ω-TA, PLP
+ +
R R 1 R * R 1
COOH COOH
3-aminocyclohexa-1, 5- Spontaneous
dienecarboxylic acid (tautomerization)
OH
COOH
Scheme 4.3 (a,b) Non-enzymatic methods to shift the equilibrium toward the product side
in ω-TA-catalyzed reactions.
Despite the significant benefit of using only a single enzyme, most TA methods
rely on alanine as amine donor, making the removal of the coproduct pyruvate a
crucial step. Consequently, different orthogonal enzyme cascades to shift the equi-
librium toward amine formation were developed (Scheme 4.4). In general, these
cascades combine two or three enzymes in a biocatalytic network. Two-enzyme