Page 90 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 90
66 4 Biocatalytic Redox Cascades Involving -Transaminases
reached a new level of efficiency [11]. Moreover, such concepts shorten the reaction
time, avoid time-consuming purification and isolation steps, and reduce thereby
the amount of organic solvents and chemicals needed. Taking these issues into
account, this chapter summarizes some recent developments employing ω-TAs in
biocatalytic redox cascades, demonstrating their potential and versatility.
4.2
General Features of -Transaminases
′
TAs or aminotransferases (E.C. 2.6.1.X) are pyridoxal-5 -phosphate (PLP) dependent
enzymes which have been identified more than half a century ago [12]. They catalyze
the reversible overall redox-neutral amino transfer from an amine donor onto a
carbonyl compound as acceptor. They can be classified as α-and ω-TAs based on the
relative position of the amino group to be transferred with respect to the carboxyl
group of the substrate [13]. While α-TAs are mainly involved in the primary nitrogen
metabolism in order to generate α-amino acids from α-keto acids, or vice versa,
ω-TAs accept a much broader substrate spectrum: these enzymes allow in principle
the conversion of any aldehyde and ketone independent of an adjacent carboxyl
group, making them consequently more interesting for synthetic applications. For
clarity, an ω-TA is defined here as an enzyme that transfers an amino group from
an amine donor onto a carbonyl moiety of the amine acceptor, wherein at least
one of the two substances is not an α-amino acid or an α-keto acid. For both types
of TAs, the cofactor PLP serves as a molecular shuttle for ammonia and electrons
between the acceptor and the donor molecule. In the catalytic cycle, PLP is initially
′
converted to pyridoxamine-5 -phosphate (PMP) at the expense of the amine donor;
PMP serves then as the intermediate amine transfer reagent for the substrate,
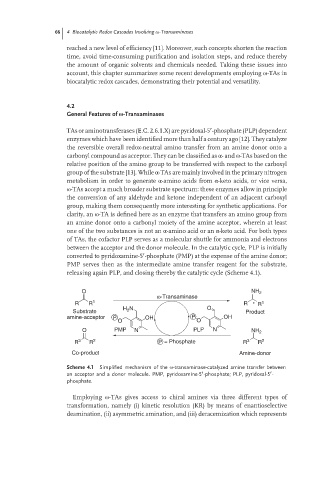
releasing again PLP, and closing thereby the catalytic cycle (Scheme 4.1).
O NH 2
ω-Transaminase
R R 1 R * R 1
H N O
Substrate 2 Product
amine-acceptor P OH P OH
O O
O PMP N PLP N NH 2
R 3 R 2 P = Phosphate R 3 R 2
Co-product Amine-donor
Scheme 4.1 Simplified mechanism of the ω-transaminase-catalyzed amine transfer between
′
an acceptor and a donor molecule. PMP, pyridoxamine-5 -phosphate; PLP, pyridoxal-5 - ′
phosphate.
Employing ω-TAs gives access to chiral amines via three different types of
transformation, namely (i) kinetic resolution (KR) by means of enantioselective
deamination, (ii) asymmetric amination, and (iii) deracemization which represents