Page 94 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 94
70 4 Biocatalytic Redox Cascades Involving -Transaminases
each individual step. Some of the recent successful developments are presented in
the following section.
4.3.1
Redox and Redox-Neutral Cascade Reactions
The direct transformation of alcohols to the corresponding amines is of growing
interest because alcohols are easily available or accessible by chemical means.
Amination of alcohols is usually catalyzed by transition metals at high temperatures
and elevated pressures. Unfortunately, there is no enzyme known today that allows
this particular functional group interconversion (FGI) in one step. Consequently, a
multi-enzyme cascade was set up for the amination of alcohols as demonstrated for
various benzylic and cinnamic alcohols under physiological conditions [24]: aerobic
alcohol oxidation toward the aldehyde was performed via a galactose oxidase
originating from Fusarium (NRRL 2903 [25]) followed by an in situ ω-TA-catalyzed
reductive amination step (Scheme 4.5).
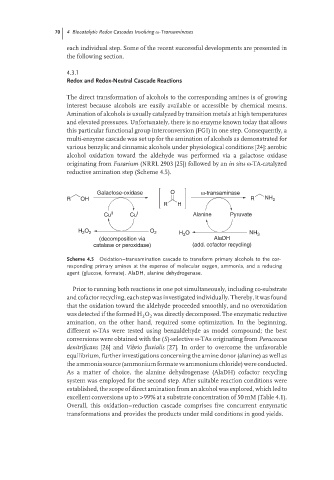
Galactose-oxidase O ω-transaminase
R OH R NH 2
R H
Cu II Cu I Alanine Pyruvate
H 2 O 2 O 2 H O NH 3
2
(decomposition via AlaDH
catalase or peroxidase) (add. cofactor recycling)
Scheme 4.5 Oxidation–transamination cascade to transform primary alcohols to the cor-
responding primary amines at the expense of molecular oxygen, ammonia, and a reducing
agent (glucose, formate). AlaDH, alanine dehydrogenase.
Prior to running both reactions in one pot simultaneously, including co-substrate
and cofactor recycling, each step was investigated individually. Thereby, it was found
that the oxidation toward the aldehyde proceeded smoothly, and no overoxidation
was detected if the formed H O was directly decomposed. The enzymatic reductive
2
2
amination, on the other hand, required some optimization. In the beginning,
different ω-TAs were tested using benzaldehyde as model compound; the best
conversions were obtained with the (S)-selective ω-TAs originating from Paracoccus
denitrificans [26] and Vibrio fluvialis [27]. In order to overcome the unfavorable
equilibrium, further investigations concerning the amine donor (alanine) as well as
the ammonia source (ammonium formate vs ammonium chloride) were conducted.
As a matter of choice, the alanine dehydrogenase (AlaDH) cofactor recycling
system was employed for the second step. After suitable reaction conditions were
established, the scope of direct amination from an alcohol was explored, which led to
excellent conversions up to >99% at a substrate concentration of 50 mM (Table 4.1).
Overall, this oxidation–reduction cascade comprises five concurrent enzymatic
transformations and provides the products under mild conditions in good yields.