Page 98 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 98
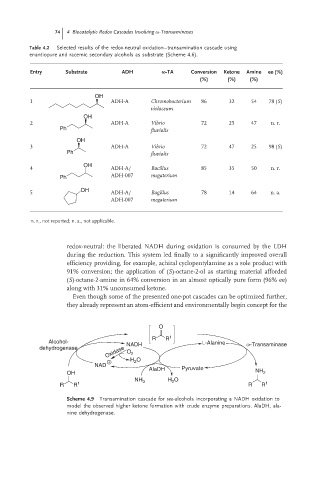
74 4 Biocatalytic Redox Cascades Involving -Transaminases
Table 4.2 Selected results of the redox-neutral oxidation–transamination cascade using
enantiopure and racemic secondary alcohols as substrate (Scheme 4.6).
Entry Substrate ADH -TA Conversion Ketone Amine ee (%)
(%) (%) (%)
OH
1 ADH-A Chromobacterium 86 32 54 78 (S)
violaceum
OH
2 ADH-A Vibrio 72 25 47 n. r.
Ph fluvialis
OH
3 ADH-A Vibrio 72 47 25 98 (S)
Ph fluvialis
OH
4 ADH-A/ Bacillus 85 35 50 n. r.
Ph ADH-007 megaterium
OH
5 ADH-A/ Bagillus 78 14 64 n. a.
ADH-007 megaterium
n. r., not reported; n. a., not applicable.
redox-neutral: the liberated NADH during oxidation is consumed by the LDH
during the reduction. This system led finally to a significantly improved overall
efficiency providing, for example, achiral cyclopentylamine as a sole product with
91% conversion; the application of (S)-octane-2-ol as starting material afforded
(S)-octane-2-amine in 64% conversion in an almost optically pure form (96% ee)
along with 31% unconsumed ketone.
Even though some of the presented one-pot cascades can be optimized further,
they already represent an atom-efficient and environmentally begin concept for the
O
R R 1
Alcohol- NADH L-Alanine ω-Transaminase
Oxidase O 2
dehydrogenase
H 2 O
NAD
AlaDH Pyruvate
OH NH 2
NH 3 H 2 O
R R 1 R R 1
Scheme 4.9 Transamination cascade for sec-alcohols incorporating a NADH oxidation to
model the observed higher ketone formation with crude enzyme preparations. AlaDH, ala-
nine dehydrogenase.