Page 101 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 101
4.3 Linear Cascade Reactions Involving ω-Transaminases 77
(S) (S)
N
Et 3 Al, LiAlH 4 H H Cl
2 h at −78 °C dr (syn/anti) 16:84
85% over two steps
(S)-Selective Pd/C, H 2 ,
ω-Transaminase (S) 4 h room temperature (S) (R)
N N
H H Cl
O Conv., >99%,
O de > 99%
D/L - Pyruvate >99% ee
94% over two steps
Alanine (recycling)
(R) (S)
Nonane-2,6-dione (R) N
Pd/C, H 2 ,
(R)-Selective N H H Cl
4 h room temperature
ω-Transaminase Conv., >99%, de > 99%
>99% ee 85% over two steps
Et 3 Al, LiAlH 4
2 h at −78 °C (R) (R)
N
H H Cl
dr (syn/anti) 18 : 82
91% over two steps
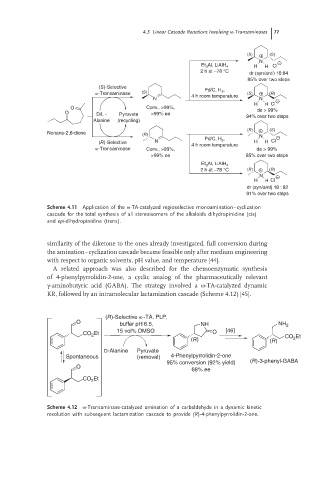
Scheme 4.11 Application of the ω-TA-catalyzed regioselective monoamination–cyclization
cascade for the total synthesis of all stereoisomers of the alkaloids dihydropinidine (cis)
and epi-dihydropinidine (trans).
similarity of the diketone to the ones already investigated, full conversion during
the amination–cyclization cascade became feasible only after medium engineering
with respect to organic solvents, pH value, and temperature [44].
A related approach was also described for the chemoenzymatic synthesis
of 4-phenylpyrrolidin-2-one, a cyclic analog of the pharmaceutically relevant
γ-aminobutyric acid (GABA). The strategy involved a ω-TA-catalyzed dynamic
KR, followed by an intramolecular lactamization cascade (Scheme 4.12) [45].
(R)-Selective ω -TA, PLP,
O buffer pH 6.5, NH NH 2
CO Et 15 vol% DMSO O [46]
2
(R) (R) CO 2 Et
D-Alanine Pyruvate
Spontaneous (removal) 4-Phenylpyrrolidin-2-one
95% conversion (92% yield) (R)-3-phenyl-GABA
O
68% ee
CO Et
2
Scheme 4.12 ω-Transaminase-catalyzed amination of a carbaldehyde in a dynamic kinetic
resolution with subsequent lactamization cascade to provide (R)-4-phenylpyrrolidin-2-one.