Page 104 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 104
80 4 Biocatalytic Redox Cascades Involving -Transaminases
4.3.4
Cascade Reactions of -TAs with Lyases and C–C Hydrolases/Lipases
In this section, we will focus on linear cascades combining ω-TAs with hydro-
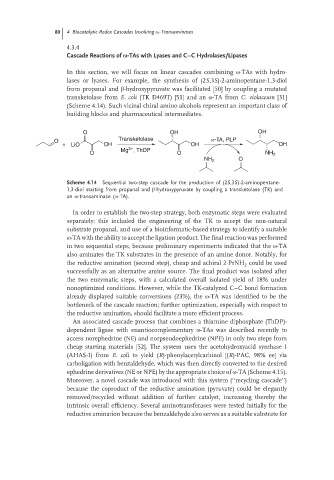
lases or lyases. For example, the synthesis of (2S,3S)-2-aminopentane-1,3-diol
from propanal and β-hydroxypyruvate was facilitated [50] by coupling a mutated
transketolase from E. coli (TK D469T) [51] and an ω-TA from C. violaceum [31]
(Scheme 4.14). Such vicinal chiral amino alcohols represent an important class of
building blocks and pharmaceutical intermediates.
O OH OH
Transketolase ω-TA, PLP
O
+ LiO OH OH OH
2+
Mg , ThDP
O O NH 2
O
NH 2
Scheme 4.14 Sequential two-step cascade for the production of (2S,3S)-2-aminopentane-
1,3-diol starting from propanal and β-hydroxypyruvate by coupling a transketolase (TK) and
an ω-transaminase (ω-TA).
In order to establish the two-step strategy, both enzymatic steps were evaluated
separately: this included the engineering of the TK to accept the non-natural
substrate propanal, and use of a bioinformatic-based strategy to identify a suitable
ω-TA with the ability to accept the ligation product. The final reaction was performed
in two sequential steps, because preliminary experiments indicated that the ω-TA
also aminates the TK substrates in the presence of an amine donor. Notably, for
the reductive amination (second step), cheap and achiral 2-PrNH could be used
2
successfully as an alternative amine source. The final product was isolated after
the two enzymatic steps, with a calculated overall isolated yield of 18% under
nonoptimized conditions. However, while the TK-catalyzed C–C bond formation
already displayed suitable conversions (23%), the ω-TA was identified to be the
bottleneck of the cascade reaction; further optimization, especially with respect to
the reductive amination, should facilitate a more efficient process.
An associated cascade process that combines a thiamine diphosphate (ThDP)-
dependent ligase with enantiocomplementary ω-TAs was described recently to
access norephedrine (NE) and norpseudoephedrine (NPE) in only two steps from
cheap starting materials [52]. The system uses the acetohydroxyacid synthase I
(AHAS-I) from E. coli to yield (R)-phenylacetylcarbinol [(R)-PAC, 98% ee] via
carboligation with benzaldehyde, which was then directly converted to the desired
ephedrine derivatives (NE or NPE) by the appropriate choice of ω-TA (Scheme 4.15).
Moreover, a novel cascade was introduced with this system (‘‘recycling cascade’’)
because the coproduct of the reductive amination (pyruvate) could be elegantly
removed/recycled without addition of further catalyst, increasing thereby the
intrinsic overall efficiency. Several aminotransferases were tested initially for the
reductive amination because the benzaldehyde also serves as a suitable substrate for