Page 106 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 106
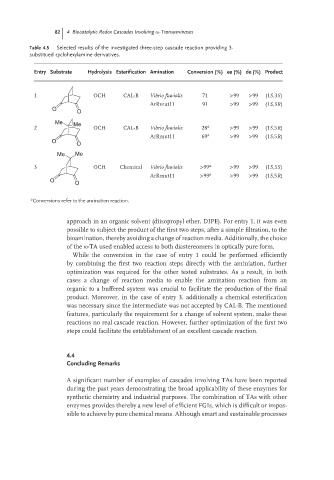
82 4 Biocatalytic Redox Cascades Involving -Transaminases
Table 4.5 Selected results of the investigated three-step cascade reaction providing 3-
substitued cyclohexylamine derivatives.
Entry Substrate Hydrolysis Esterification Amination Conversion (%) ee (%) de (%) Product
1 OCH CAL-B Vibrio fluvialis 71 >99 >99 (1S,3S)
ArRmut11 91 >99 >99 (1S,3R)
O
O
Me Me
2 OCH CAL-B Vibrio fluvialis 28 a >99 >99 (1S,5R)
ArRmut11 69 a >99 >99 (1S,5R)
O
O
Me Me
3 OCH Chemical Vibrio fluvialis >99 a >99 >99 (1S,5S)
ArRmut11 >99 a >99 >99 (1S,5R)
O
O
a Conversions refer to the amination reaction.
approach in an organic solvent (diisopropyl ether, DIPE). For entry 1, it was even
possible to subject the product of the first two steps, after a simple filtration, to the
bioamination, thereby avoiding a change of reaction media. Additionally, the choice
of the ω-TA used enabled access to both diastereomers in optically pure form.
While the conversion in the case of entry 1 could be performed efficiently
by combining the first two reaction steps directly with the aminiation, further
optimization was required for the other tested substrates. As a result, in both
cases a change of reaction media to enable the amination reaction from an
organic to a buffered system was crucial to facilitate the production of the final
product. Moreover, in the case of entry 3, additionally a chemical esterification
was necessary since the intermediate was not accepted by CAL-B. The mentioned
features, particularly the requirement for a change of solvent system, make these
reactions no real cascade reaction. However, further optimization of the first two
steps could facilitate the establishment of an excellent cascade reaction.
4.4
Concluding Remarks
A significant number of examples of cascades involving TAs have been reported
during the past years demonstrating the broad applicability of these enzymes for
synthetic chemistry and industrial purposes. The combination of TAs with other
enzymes provides thereby a new level of efficient FGIs, which is difficult or impos-
sible to achieve by pure chemical means. Although smart and sustainable processes