Page 96 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 96
72 4 Biocatalytic Redox Cascades Involving -Transaminases
and 3-phenyl-1-propanol, for example, were fully converted to the respective amines
as the sole products; the transformations of cinnamyl or benzyl alcohol, however,
provided significant amounts of the amines (70–87%), but the intermediate
aldehyde was also found in up to 30% at full conversion.
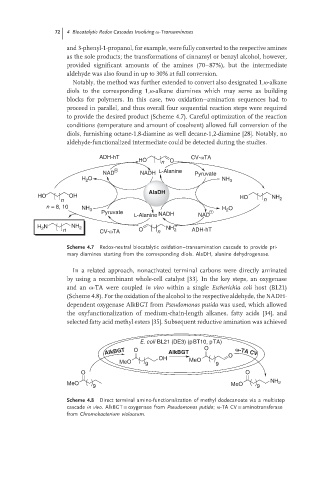
Notably, the method was further extended to convert also designated 1,ω-alkane
diols to the corresponding 1,ω-alkane diamines which may serve as building
blocks for polymers. In this case, two oxidation–amination sequences had to
proceed in parallel, and thus overall four sequential reaction steps were required
to provide the desired product (Scheme 4.7). Careful optimization of the reaction
conditions (temperature and amount of cosolvent) allowed full conversion of the
diols, furnishing octane-1,8-diamine as well decane-1,2-diamine [28]. Notably, no
aldehyde-functionalized intermediate could be detected during the studies.
ADH-hT CV-ωTA
HO n O
NAD NADH L-Alanine Pyruvate
H O NH 3
2
AlaDH
HO OH HO NH
n n 2
n = 8, 10 H O
NH 3 2
Pyruvate NADH
L-Alanine NAD
H N n NH 2 CV-ωTA O n NH 2 ADH-hT
2
Scheme 4.7 Redox-neutral biocatalytic oxidation–transamination cascade to provide pri-
mary diamines starting from the corresponding diols. AlaDH, alanine dehydrogenase.
In a related approach, nonactivated terminal carbons were directly aminated
by using a recombinant whole-cell catalyst [33]. In the key steps, an oxygenase
and an ω-TA were coupled in vivo within a single Escherichia coli host (BL21)
(Scheme 4.8). For the oxidation of the alcohol to the respective aldehyde, the NADH-
dependent oxygenase AlkBGT from Pseudomonas putida was used, which allowed
the oxyfunctionalization of medium-chain-length alkanes, fatty acids [34], and
selected fatty acid methyl esters [35]. Subsequent reductive amination was achieved
E. coli BL21 (DE3) (pBT10, pTA)
AlkBGT O AlkBGT O ω-TA CV
OH O
MeO MeO
9 9
O O
NH
MeO MeO 2
9 9
Scheme 4.8 Direct terminal amino-functionalization of methyl dodecanoate via a multistep
cascade in vivo.AlkBGT = oxygenase from Pseudomonas putida; ω-TA CV = aminotransferase
from Chromobacterium violaceum.