Page 104 - Biodegradable Polyesters
P. 104
82 4 Synthesis, Properties, and Mathematical Modeling of Biodegradable Aliphatic Polyesters
∘
58 C [28]. These values for PPSeb and PPSu are lower than those of correspond-
ing polyesters containing ethylene glycol or butanediol instead of propanediol.
For example, it was found that poly(ethylene sebacate) (PESeb) and poly(butylene
∘
0
sebacate) (PBSeb) have T at 90.2 and 77.4 C, respectively, [27] while the T m 0
m
∘
values obtained for PESu and PBSu are 117 and 133.5 C, respectively [28]. This
behavior is due to the odd–even effect of methylene groups on melting points,
which also affects the crystallization rates of aliphatic polyesters prepared from
1,3-PD.
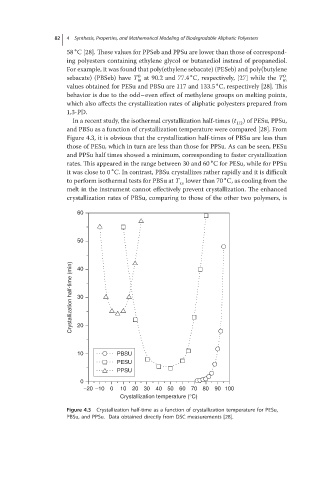
In a recent study, the isothermal crystallization half-times (t 1/2 ) of PESu, PPSu,
and PBSu as a function of crystallization temperature were compared [28]. From
Figure 4.3, it is obvious that the crystallization half-times of PBSu are less than
those of PESu, which in turn are less than those for PPSu. As can be seen, PESu
and PPSu half times showed a minimum, corresponding to faster crystallization
∘
rates. This appeared in the range between 30 and 60 Cfor PESu,while forPPSu
∘
it was close to 0 C. In contrast, PBSu crystallizes rather rapidly and it is difficult
∘
to perform isothermal tests for PBSu at T lower than 70 C, as cooling from the
cs
melt in the instrument cannot effectively prevent crystallization. The enhanced
crystallization rates of PBSu, comparing to those of the other two polymers, is
60
50
Crystallization half-time (min) 30
40
20
10 PBSU
PESU
PPSU
0
−20 −10 0 10 20 30 40 50 60 70 80 90 100
Crystallization temperature (°C)
Figure 4.3 Crystallization half-time as a function of crystallization temperature for PESu,
PBSu, and PPSu. Data obtained directly from DSC measurements [28].