Page 159 - Biodegradable Polyesters
P. 159
6.2 Shape Memory Polymer Systems 137
70
Heating
D
60
50 T c
Strain (%) 40 Cooling C
30
20
10
0 Deformation 1000
–20 800
0 A Heating 600
20 400
40 B 200
60 0 Stress (kPa)
80
Temperature (°C)
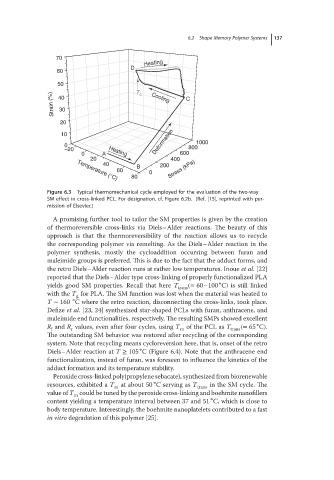
Figure 6.3 Typical thermomechanical cycle employed for the evaluation of the two-way
SM effect in cross-linked PCL. For designation, cf. Figure 6.2b. (Ref. [15], reprinted with per-
mission of Elsevier.)
A promising further tool to tailor the SM properties is given by the creation
of thermoreversible cross-links via Diels–Alder reactions. The beauty of this
approach is that the thermoreversibility of the reaction allows us to recycle
the corresponding polymer via remelting. As the Diels–Alder reaction in the
polymer synthesis, mostly the cycloaddition occurring between furan and
maleimide groups is preferred. This is due to the fact that the adduct forms, and
the retro Diels–Alder reaction runs at rather low temperatures. Inoue et al. [22]
reported that the Diels–Alder type cross-linking of properly functionalized PLA
∘
yields good SM properties. Recall that here T trans (= 60–100 C) is still linked
with the T for PLA. The SM function was lost when the material was heated to
g
∘
T ∼ 160 C where the retro reaction, disconnecting the cross-links, took place.
Defize et al. [23, 24] synthesized star-shaped PCLs with furan, anthracene, and
maleimide end functionalities, respectively. The resulting SMPs showed excellent
∘
R and R values, even after four cycles, using T of the PCL as T (= 65 C).
f r m trans
The outstanding SM behavior was restored after recycling of the corresponding
system. Note that recycling means cycloreversion here, that is, onset of the retro
∘
Diels–Alder reaction at T ≥ 105 C (Figure 6.4). Note that the anthracene end
functionalization, instead of furan, was foreseen to influence the kinetics of the
adduct formation and its temperature stability.
Peroxide cross-linked poly(propylene sebacate), synthesized from biorenewable
∘
resources, exhibited a T at about 50 C serving as T in the SM cycle. The
m trans
value of T could be tuned by the peroxide cross-linking and boehmite nanofillers
m ∘
content yielding a temperature interval between 37 and 51 C, which is close to
body temperature. Interestingly, the boehmite nanoplatelets contributed to a fast
in vitro degradation of this polymer [25].