Page 160 - Biodegradable Polyesters
P. 160
138 6 Shape Memory Systems with Biodegradable Polyesters
180 180
160 160
140 140
Elongation (%) 100 1st cycle Elongation (%) 100 1st cycle
120
120
2nd cycle
2nd cycle
80
80
3rd cycle
3rd cycle
60
40 4th cycle 60 4th cycle
40
20 20
0.6 0.6
0 0.5 0 0.5
10 0.4 10 0.4
20 20
30 0.3 30 0.3
40 0.2 40 0.2
50 0.1 Stress (MPa) 50 0.1 Stress (MPa)
60 0.0 Temperature (°C) 60 0.0
(a) (b)
Temperature (°C)
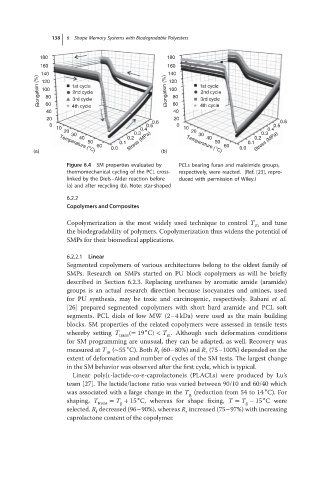
Figure 6.4 SM properties evaluated by PCLs bearing furan and maleimide groups,
thermomechanical cycling of the PCL cross- respectively, were reacted. (Ref. [23], repro-
linked by the Diels–Alder reaction before duced with permission of Wiley.)
(a) and after recycling (b). Note: star-shaped
6.2.2
Copolymers and Composites
Copolymerization is the most widely used technique to control T m and tune
the biodegradability of polymers. Copolymerization thus widens the potential of
SMPs for their biomedical applications.
6.2.2.1 Linear
Segmented copolymers of various architectures belong to the oldest family of
SMPs. Research on SMPs started on PU block copolymers as will be briefly
described in Section 6.2.3. Replacing urethanes by aromatic amide (aramide)
groups is an actual research direction because isocyanates and amines, used
for PU synthesis, may be toxic and carcinogenic, respectively. Rabani et al.
[26] prepared segmented copolymers with short hard aramide and PCL soft
segments. PCL diols of low MW (2–4 kDa) were used as the main building
blocks. SM properties of the related copolymers were assessed in tensile tests
∘
whereby setting T trans (= 19 C) < T . Although such deformation conditions
m
for SM programming are unusual, they can be adapted, as well. Recovery was
∘
measured at T (∼55 C). Both R (60–80%) and R (75–100%) depended on the
m f r
extent of deformation and number of cycles of the SM tests. The largest change
in the SM behavior was observed after the first cycle, which is typical.
Linear poly(L-lactide-co-ε-caprolactone)s (PLACLs) were produced by Lu’s
team [27]. The lactide/lactone ratio was varied between 90/10 and 60/40 which
∘
was associated with a large change in the T (reduction from 54 to 14 C). For
g
∘ ∘
shaping, T trans = T + 15 C, whereas for shape fixing, T = T − 15 Cwere
g
g
selected. R decreased (96–90%), whereas R increased (75–97%) with increasing
f
r
caprolactone content of the copolymer.