Page 246 - Biodegradable Polyesters
P. 246
224 9 Environment-Friendly Methods for Converting Biodegradable Polyesters
(a) (b)
64 nm
144 nm
21 nm
500 nm 1 μm
(c) (d)
500 μm 10 μm
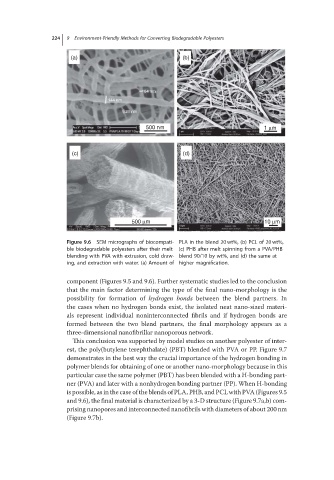
Figure 9.6 SEM micrographs of biocompati- PLA in the blend 20 wt%, (b) PCL of 20 wt%,
ble biodegradable polyesters after their melt (c) PHB after melt spinning from a PVA/PHB
blending with PVA with extrusion, cold draw- blend 90/10 by wt%, and (d) the same at
ing, and extraction with water. (a) Amount of higher magnification.
component (Figures 9.5 and 9.6). Further systematic studies led to the conclusion
that the main factor determining the type of the final nano-morphology is the
possibility for formation of hydrogen bonds between the blend partners. In
the cases when no hydrogen bonds exist, the isolated neat nano-sized materi-
als represent individual noninterconnected fibrils and if hydrogen bonds are
formed between the two blend partners, the final morphology appears as a
three-dimensional nanofibrillar nanoporous network.
This conclusion was supported by model studies on another polyester of inter-
est, the poly(butylene terephthalate) (PBT) blended with PVA or PP. Figure 9.7
demonstrates in the best way the crucial importance of the hydrogen bonding in
polymer blends for obtaining of one or another nano-morphology because in this
particular case the same polymer (PBT) has been blended with a H-bonding part-
ner (PVA) and later with a nonhydrogen bonding partner (PP). When H-bonding
is possible, as in the case of the blends of PLA, PHB, and PCL with PVA (Figures 9.5
and 9.6), the final material is characterized by a 3-D structure (Figure 9.7a,b) com-
prising nanopores and interconnected nanofibrils with diameters of about 200 nm
(Figure 9.7b).