Page 247 - Biodegradable Polyesters
P. 247
9.3 Effect of Hydrogen Bonding in Polymer Blends on Nano-Morphology 225
(a) (b)
205 nm
306 nm
200 nm
20 μm 20 μm 500 nm 500 nm
(c) (d)
231 nm
252 nm
227 nm
20 μm 500 nm 500 nm
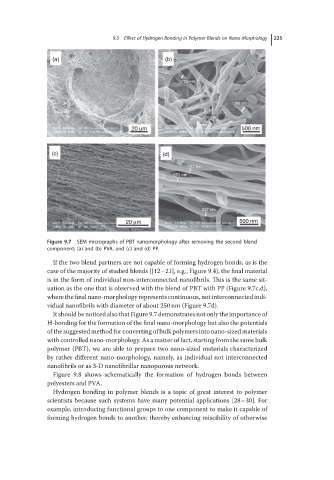
Figure 9.7 SEM micrographs of PBT nanomorphology after removing the second blend
component: (a) and (b) PVA, and (c) and (d) PP.
If the two blend partners are not capable of forming hydrogen bonds, as is the
case of the majority of studied blends ([12–21], e.g., Figure 9.4), the final material
is in the form of individual non-interconnected nanofibrils. This is the same sit-
uation as the one that is observed with the blend of PBT with PP (Figure 9.7c,d),
where the final nano-morphology represents continuous, not interconnected indi-
vidual nanofibrils with diameter of about 250 nm (Figure 9.7d).
It should be noticed also that Figure 9.7 demonstrates not only the importance of
H-bonding for the formation of the final nano-morphology but also the potentials
of the suggested method for converting of bulk polymers into nano-sized materials
with controlled nano-morphology. As a matter of fact, starting from the same bulk
polymer (PBT), we are able to prepare two nano-sized materials characterized
by rather different nano-morphology, namely, as individual not interconnected
nanofibrils or as 3-D nanofibrillar nanoporous network.
Figure 9.8 shows schematically the formation of hydrogen bonds between
polyesters and PVA.
Hydrogen bonding in polymer blends is a topic of great interest to polymer
scientists because such systems have many potential applications [28–30]. For
example, introducing functional groups to one component to make it capable of
forming hydrogen bonds to another, thereby enhancing miscibility of otherwise