Page 50 - Biodegradable Polyesters
P. 50
28 2 Functional (Bio)degradable Polyesters by Radical Ring-Opening Polymerization
2.2.2
Radical Ring-Opening Polymerization Mechanism
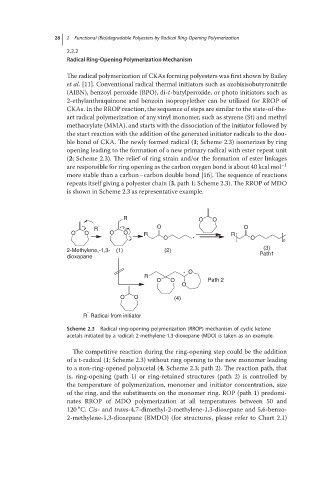
The radical polymerization of CKAs forming polyesters was first shown by Bailey
et al. [11]. Conventional radical thermal initiators such as azobisisobutyronitrile
(AIBN), benzoyl peroxide (BPO), di-t-butylperoxide, or photo initiators such as
2-ethylanthraquinone and benzoin isopropylether can be utilized for RROP of
CKAs. In the RROP reaction, the sequence of steps are similar to the state-of-the-
art radical polymerization of any vinyl monomer, such as styrene (St) and methyl
methacrylate (MMA), and starts with the dissociation of the initiator followed by
the start reaction with the addition of the generated initiator radicals to the dou-
ble bond of CKA. The newly formed radical (1; Scheme 2.3) isomerizes by ring
opening leading to the formation of a new primary radical with ester repeat unit
(2; Scheme 2.3). The relief of ring strain and/or the formation of ester linkages
are responsible for ring opening as the carbon oxygen bond is about 40 kcal mol −1
more stable than a carbon–carbon double bond [16]. The sequence of reactions
repeats itself giving a polyester chain (3, path 1; Scheme 2.3). The RROP of MDO
is shown in Scheme 2.3 as representative example.
R O O
.
R O O
O O O O R R
O O
n
(3)
2-Methylene,-1,3- (1) (2)
dioxapane Path1
O
R
O O Path 2
O
O O (4)
.
R Radical from initiator
Scheme 2.3 Radical ring-opening polymerization (RROP) mechanism of cyclic ketene
acetals initiated by a radical; 2-methylene-1,3-dioxepane (MDO) is taken as an example.
The competitive reaction during the ring-opening step could be the addition
of a t-radical (1; Scheme 2.3) without ring opening to the new monomer leading
to a non-ring-opened polyacetal (4, Scheme 2.3; path 2). The reaction path, that
is, ring-opening (path 1) or ring-retained structures (path 2) is controlled by
the temperature of polymerization, monomer and initiator concentration, size
of the ring, and the substituents on the monomer ring. ROP (path 1) predomi-
nates RROP of MDO polymerization at all temperatures between 50 and
∘
120 C. Cis-and trans-4,7-dimethyl-2-methylene-1,3-dioxepane and 5,6-benzo-
2-methylene-1,3-dioxepane (BMDO) (for structures, please refer to Chart 2.1)