Page 51 - Biodegradable Polyesters
P. 51
2.2 Radical Ring-Opening Polymerization (RROP) of Cyclic Ketene Acetals 29
are other seven-membered CKAs undergoing quantitative ring-opening radical
∘
reaction at 120 C forming corresponding polyesters [17–19]. The driving force
for the reaction is the relief of ring strain and the formation of a stable ester
bond.
In contrast, a stable five-membered monomer 2-methylene-1,3-dioxolane
gives a mixture of ring-opened and ring-retained structures at all temperatures
of polymerizations due to the formation of a primary unstable radical after
the ring-opening reaction. The ratio of ring-opened/ring-retained structure
∘
increases with the increase in temperature (50% ring opening at 60 C and 83% at
∘
125 C) and with decrease in the monomer concentration. The radical-stabilizing
group onto the ring plays a crucial role in quantitative ring opening for the
formation of polyesters. The radical formed after ring-opening reaction could
be made more stable by monomer designing. For example, the introduction
of a phenyl substituent at fourth position of 2-methylene-1,3-dioxolane gave
2-methylene-4-phenyl-1,3-dioxolane which was shown to undergo quantitative
∘
and regioselective ring opening at all temperatures from 60 to 150 Ctogive
polyester, poly[γ-(β-phenyl)butyrolactone][20].
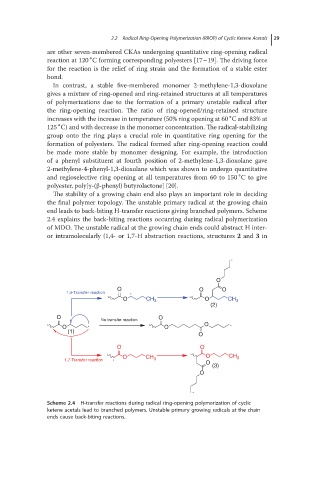
The stability of a growing chain end also plays an important role in deciding
the final polymer topology. The unstable primary radical at the growing chain
end leads to back-biting H-transfer reactions giving branched polymers. Scheme
2.4 explains the back-biting reactions occurring during radical polymerization
of MDO. The unstable radical at the growing chain ends could abstract H inter-
or intramolecularly (1,4- or 1,7-H abstraction reactions, structures 2 and 3 in
O
O O O
1,4-Transfer reaction
O CH 3 O CH 3
(2)
O O
No transfer reaction
O
O O
(1)
O
O O
O CH 3 O CH 3
1,7-Transfer reaction
O
(3)
O
Scheme 2.4 H-transfer reactions during radical ring-opening polymerization of cyclic
ketene acetals lead to branched polymers. Unstable primary growing radicals at the chain
ends cause back-biting reactions.