Page 55 - Biodegradable Polyesters
P. 55
2.2 Radical Ring-Opening Polymerization (RROP) of Cyclic Ketene Acetals 33
O O O Mix and heat
O
Donor Acceptor
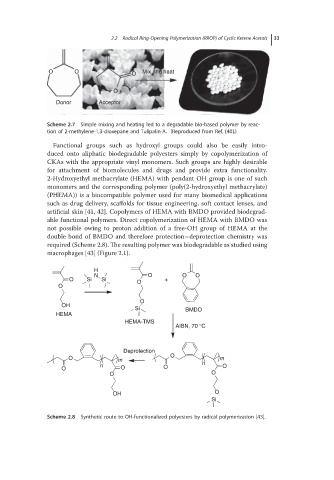
Scheme 2.7 Simple mixing and heating led to a degradable bio-based polymer by reac-
tion of 2-methylene-1,3-dioxepane and Tulipalin-A. (Reproduced from Ref. [40].)
Functional groups such as hydroxyl groups could also be easily intro-
duced onto aliphatic biodegradable polyesters simply by copolymerization of
CKAs with the appropriate vinyl monomers. Such groups are highly desirable
for attachment of biomolecules and drugs and provide extra functionality.
2-Hydroxyethyl methacrylate (HEMA) with pendant OH group is one of such
monomers and the corresponding polymer (poly(2-hydroxyethyl methacrylate)
(PHEMA)) is a biocompatible polymer used for many biomedical applications
such as drug delivery, scaffolds for tissue engineering, soft contact lenses, and
artificial skin [41, 42]. Copolymers of HEMA with BMDO provided biodegrad-
able functional polymers. Direct copolymerization of HEMA with BMDO was
not possible owing to proton addition of a free-OH group of HEMA at the
double bond of BMDO and therefore protection–deprotection chemistry was
required (Scheme 2.8). The resulting polymer was biodegradable as studied using
macrophages [43] (Figure 2.1).
H
N O O O
O Si Si +
O
O
O
OH
Si BMDO
HEMA
HEMA-TMS
AIBN, 70 °C
Deprotection
O
O m m
n n O
O O O
O O
OH O
Si
Scheme 2.8 Synthetic route to OH-functionalized polyesters by radical polymerization [43].