Page 56 - Biodegradable Polyesters
P. 56
34 2 Functional (Bio)degradable Polyesters by Radical Ring-Opening Polymerization
a b c
(A)
100
1 2 Mn = 6 kDa
80
Relative mass (%) 60
40
20
0
Original film With low cell With high cell 8 9 10 11 12 13
(B) concentration concentration (C) Retention volume (mL)
4
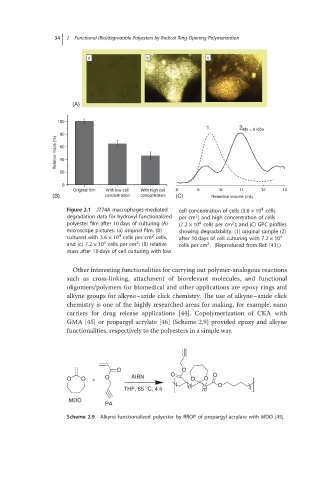
Figure 2.1 J774A macrophages-mediated cell concentration of cells (3.6 × 10 cells
degradation data for hydroxyl-functionalized per cm ) and high concentration of cells
2
polyester film after 10 days of culturing (A) (7.2 × 10 cells per cm ); and (C) GPC profiles
4
2
microscope pictures: (a) original film, (b) showing degradability; (1) original sample (2)
2
4
cultured with 3.6 × 10 cells per cm cells, after 10 days of cell culturing with 7.2 × 10 4
4
2
and (c) 7.2 × 10 cells per cm ; (B) relative cells per cm . (Reproduced from Ref. [43].)
2
mass after 10 days of cell culturing with low
Other interesting functionalities for carrying out polymer-analogous reactions
such as cross-linking, attachment of biorelevant molecules, and functional
oligomers/polymers for biomedical and other applications are epoxy rings and
alkyne groups for alkyne–azide click chemistry. The use of alkyne–azide click
chemistry is one of the highly researched areas for making, for example, nano
carriers for drug release applications [44]. Copolymerization of CKA with
GMA [45] or propargyl acrylate [46] (Scheme 2.9) provided epoxy and alkyne
functionalities, respectively to the polyesters in a simple way.
O O
O O + O AIBN O O O O
n O
THF, 65 °C, 4 h m l
MDO
PA
Scheme 2.9 Alkyne-functionalized polyester by RROP of propargyl acrylate with MDO [45].