Page 61 - Biodegradable Polyesters
P. 61
2.2 Radical Ring-Opening Polymerization (RROP) of Cyclic Ketene Acetals 39
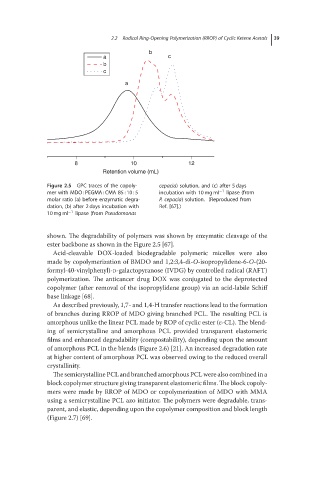
b
a c
b
c
a
8 10 12
Retention volume (mL)
Figure 2.5 GPC traces of the copoly- cepacia) solution, and (c) after 5 days
mer with MDO :PEGMA :CMA 85:10:5 incubation with 10 mg ml −1 lipase (from
molar ratio (a) before enzymatic degra- P. cepacia) solution. (Reproduced from
dation, (b) after 2 days incubation with Ref. [67].)
10 mg ml −1 lipase (from Pseudomonas
shown. The degradability of polymers was shown by enzymatic cleavage of the
ester backbone as shown in the Figure 2.5 [67].
Acid-cleavable DOX-loaded biodegradable polymeric micelles were also
made by copolymerization of BMDO and 1,2:3,4-di-O-isopropylidene-6-O-(20-
formyl-40-vinylphenyl)-D-galactopyranose (IVDG) by controlled radical (RAFT)
polymerization. The anticancer drug DOX was conjugated to the deprotected
copolymer (after removal of the isopropylidene group) via an acid-labile Schiff
base linkage [68].
As described previously, 1,7- and 1,4-H transfer reactions lead to the formation
of branches during RROP of MDO giving branched PCL. The resulting PCL is
amorphous unlike the linear PCL made by ROP of cyclic ester (ε-CL). The blend-
ing of semicrystalline and amorphous PCL provided transparent elastomeric
films and enhanced degradability (compostability), depending upon the amount
of amorphous PCL in the blends (Figure 2.6) [21]. An increased degradation rate
at higher content of amorphous PCL was observed owing to the reduced overall
crystallinity.
The semicrystalline PCL and branched amorphous PCL were also combined in a
block copolymer structure giving transparent elastomeric films. The block copoly-
mers were made by RROP of MDO or copolymerization of MDO with MMA
using a semicrystalline PCL azo initiator. The polymers were degradable, trans-
parent, and elastic, depending upon the copolymer composition and block length
(Figure 2.7) [69].