Page 196 - Biofuels Refining and Performance
P. 196
Processing of Vegetable Oils as Biodiesel and Engine Performance 179
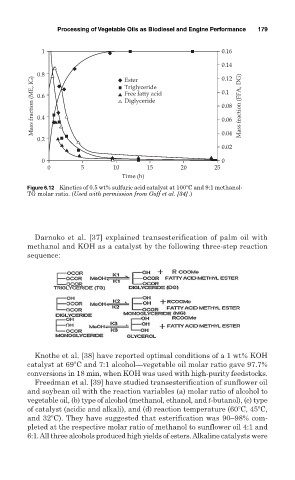
1 0.16
0.14
Mass fraction (ME, IG) 0.8 Triglyceride 0.1 Mass fraction (FFA, DG)
0.12
Ester
Free fatty acid
0.6
Diglyceride
0.08
0.4
0.06
0.2 0.04
0.02
0 0
0 5 10 15 20 25
Time (h)
Figure 6.12 Kinetics of 0.5 wt% sulfuric acid catalyst at 100 C and 9:1 methanol-
TG molar ratio. (Used with permission from Goff et al. [34].)
Darnoko et al. [37] explained transesterification of palm oil with
methanol and KOH as a catalyst by the following three-step reaction
sequence:
Knothe et al. [38] have reported optimal conditions of a 1 wt% KOH
catalyst at 69 C and 7:1 alcohol—vegetable oil molar ratio gave 97.7%
conversions in 18 min, when KOH was used with high-purity feedstocks.
Freedman et al. [39] have studied transesterification of sunflower oil
and soybean oil with the reaction variables (a) molar ratio of alcohol to
vegetable oil, (b) type of alcohol (methanol, ethanol, and t-butanol), (c) type
of catalyst (acidic and alkali), and (d) reaction temperature (60 C, 45 C,
and 32 C). They have suggested that esterification was 90–98% com-
pleted at the respective molar ratio of methanol to sunflower oil 4:1 and
6:1. All three alcohols produced high yields of esters. Alkaline catalysts were