Page 197 - Biofuels Refining and Performance
P. 197
180 Chapter Six
generally much more effective than acid catalysts. The reaction was
performed successfully at both 45 C and 60 C in 4 h, with the production
of 97% of ME.
Kruclen et al. [40] have presented a process for conversion of a high-
melting point palm oil fraction into ethyl esters, which could be used as a
diesel fuel substitute. The amount of catalyst used (KOH) was 0.1–1%, and
the reaction was completed rapidly at 80 C with yields of 80–94%, depend-
ing on the concentration of catalysts. The specific gravity of ethyl ester
varied from 0.847 to 0.864 with kinematic viscosity of 4.4–4.6 cSt at 40 C.
Gelbard et al. [41] have determined the yield of transesterification of
1
rapeseed oil with methanol and base by H-NMR (nuclear magnetic
resonance) spectroscopy. The relevant signals chosen for integration
are those of methoxy groups in ME at 3.7 ppm (parts per million) (sin-
glet) and of the -carbonyl methylene groups present in all fatty ester
derivatives at 2.3 ppm. The latter appears as a triplet, so accurate meas-
urements require good separation of this multiple at 2.1 ppm, which is
related to allylic protons.
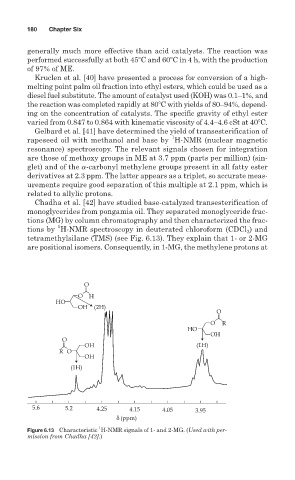
Chadha et al. [42] have studied base-catalyzed transesterification of
monoglycerides from pongamia oil. They separated monoglyceride frac-
tions (MG) by column chromatography and then characterized the frac-
1
tions by H-NMR spectroscopy in deuterated chloroform (CDCl ) and
3
tetramethylsilane (TMS) (see Fig. 6.13). They explain that 1- or 2-MG
are positional isomers. Consequently, in 1-MG, the methylene protons at
O
O H
HO
OH (2H)
O
O R
HO
OH
O
OH (1H)
R O
OH
(1H)
5.6 5.2 4.25 4.15 4.05 3.95
δ (ppm)
1
Figure 6.13 Characteristic H-NMR signals of 1- and 2-MG. (Used with per-
mission from Chadha [42].)