Page 240 - Biofuels Refining and Performance
P. 240
Cracking of Lipids for Fuels and Chemicals 223
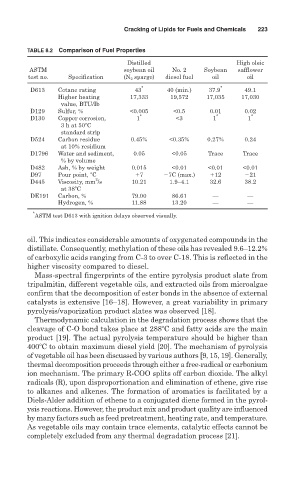
TABLE 8.2 Comparison of Fuel Properties
Distilled High oleic
ASTM soybean oil No. 2 Soybean safflower
test no. Specification (N 2 sparge) diesel fuel oil oil
D613 Cetane rating 43 * 40 (min.) 37.9 * 49.1
Higher heating 17,333 19,572 17,035 17,030
value, BTU/Ib
D129 Sulfur, % <0.005 <0.5 0.01 0.02
D130 Copper corrosion, 1 * <3 1 * 1 *
3 h at 50 C
standard strip
D524 Carbon residue 0.45% <0.35% 0.27% 0.24
at 10% residium
D1796 Water and sediment, 0.05 <0.05 Trace Trace
% by volume
D482 Ash, % by weight 0.015 <0.01 <0.01 <0.01
D97 Pour point, C 7 7C (max.) 12 21
2
D445 Viscosity, mm /s 10.21 1.9–4.1 32.6 38.2
at 38 C
DE191 Carbon, % 79.00 86.61 — —
Hydrogen, % 11.88 13.20 — —
*
ASTM test D613 with ignition delays observed visually.
oil. This indicates considerable amounts of oxygenated compounds in the
distillate. Consequently, methylation of these oils has revealed 9.6–12.2%
of carboxylic acids ranging from C-3 to over C-18. This is reflected in the
higher viscosity compared to diesel.
Mass-spectral fingerprints of the entire pyrolysis product slate from
tripalmitin, different vegetable oils, and extracted oils from microalgae
confirm that the decomposition of ester bonds in the absence of external
catalysts is extensive [16–18]. However, a great variability in primary
pyrolysis/vaporization product slates was observed [18].
Thermodynamic calculation in the degradation process shows that the
cleavage of C-O bond takes place at 288 C and fatty acids are the main
product [19]. The actual pyrolysis temperature should be higher than
400 C to obtain maximum diesel yield [20]. The mechanism of pyrolysis
of vegetable oil has been discussed by various authors [9, 15, 19]. Generally,
thermal decomposition proceeds through either a free-radical or carbonium
ion mechanism. The primary R-COO splits off carbon dioxide. The alkyl
radicals (R), upon disproportionation and elimination of ethene, give rise
to alkanes and alkenes. The formation of aromatics is facilitated by a
Diels-Alder addition of ethene to a conjugated diene formed in the pyrol-
ysis reactions. However, the product mix and product quality are influenced
by many factors such as feed pretreatment, heating rate, and temperature.
As vegetable oils may contain trace elements, catalytic effects cannot be
completely excluded from any thermal degradation process [21].