Page 244 - Biofuels Refining and Performance
P. 244
Cracking of Lipids for Fuels and Chemicals 227
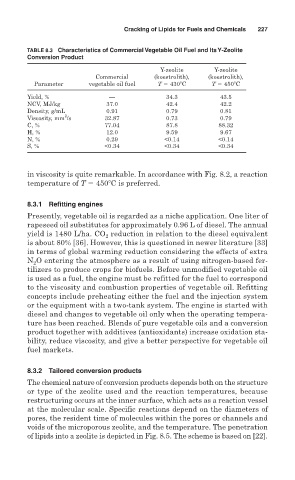
TABLE 8.3 Characteristics of Commercial Vegetable Oil Fuel and Its Y-Zeolite
Conversion Product
Y-zeolite Y-zeolite
Commercial (koestrolith), (koestrolith),
Parameter vegetable oil fuel T 430 C T 450 C
Yield, % — 34.3 43.5
NCV, MJ/kg 37.0 42.4 42.2
Density, g/mL 0.91 0.79 0.81
2
Viscosity, mm /s 32.87 0.73 0.79
C, % 77.04 87.8 88.32
H, % 12.0 9.59 9.67
N, % 0.29 <0.14 <0.14
S, % <0.34 <0.34 <0.34
in viscosity is quite remarkable. In accordance with Fig. 8.2, a reaction
temperature of T 450 C is preferred.
8.3.1 Refitting engines
Presently, vegetable oil is regarded as a niche application. One liter of
rapeseed oil substitutes for approximately 0.96 L of diesel. The annual
yield is 1480 L/ha. CO 2 reduction in relation to the diesel equivalent
is about 80% [36]. However, this is questioned in newer literature [33]
in terms of global warming reduction considering the effects of extra
N 2 O entering the atmosphere as a result of using nitrogen-based fer-
tilizers to produce crops for biofuels. Before unmodified vegetable oil
is used as a fuel, the engine must be refitted for the fuel to correspond
to the viscosity and combustion properties of vegetable oil. Refitting
concepts include preheating either the fuel and the injection system
or the equipment with a two-tank system. The engine is started with
diesel and changes to vegetable oil only when the operating tempera-
ture has been reached. Blends of pure vegetable oils and a conversion
product together with additives (antioxidants) increase oxidation sta-
bility, reduce viscosity, and give a better perspective for vegetable oil
fuel markets.
8.3.2 Tailored conversion products
The chemical nature of conversion products depends both on the structure
or type of the zeolite used and the reaction temperatures, because
restructuring occurs at the inner surface, which acts as a reaction vessel
at the molecular scale. Specific reactions depend on the diameters of
pores, the resident time of molecules within the pores or channels and
voids of the microporous zeolite, and the temperature. The penetration
of lipids into a zeolite is depicted in Fig. 8.5. The scheme is based on [22].