Page 30 - Biofuels Refining and Performance
P. 30
Energy and Its Biological Resources 13
But in lactic fermentation from polysaccharide,
(Glucosyl) → 2[CH CHOHCOOH] (Glucosyl) n 1 52,000 cal
3
n
CHOHCOOH 3O → 3CO 3H O 319,500 cal
CH 3 2 2 2
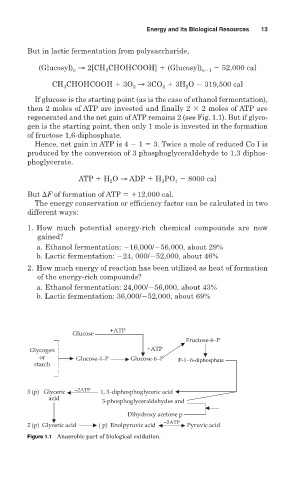
If glucose is the starting point (as is the case of ethanol fermentation),
then 2 moles of ATP are invested and finally 2 2 moles of ATP are
regenerated and the net gain of ATP remains 2 (see Fig. 1.1). But if glyco-
gen is the starting point, then only 1 mole is invested in the formation
of fructose 1,6-diphosphate.
Hence, net gain in ATP is 4 1 3. Twice a mole of reduced Co I is
produced by the conversion of 3 phosphoglyceraldehyde to 1,3 diphos-
phoglycerate.
ATP H O → ADP H PO 8000 cal
4
3
2
But F of formation of ATP 12,000 cal.
The energy conservation or efficiency factor can be calculated in two
different ways:
1. How much potential energy-rich chemical compounds are now
gained?
a. Ethanol fermentation: 16,000/ 56,000, about 29%
b. Lactic fermentation: 24, 000/ 52,000, about 46%
2. How much energy of reaction has been utilized as heat of formation
of the energy-rich compounds?
a. Ethanol fermentation: 24,000/ 56,000, about 43%
b. Lactic fermentation: 36,000/ 52,000, about 69%
+ATP
Glucose
Fructose-6–P
Glycogen +ATP
or
Glucose–l–P Glucose-6–P F-1–6-diphosphate
starch
3 (p) Glyceric –2ATP 1, 3-diphosphoglyceric acid
acid
3-phosphoglyceraldehydes and
Dihydroxy acetone p
−2ATP
2 (p) Glyceric acid ( p) Enolpyruvic acid Pyruvic acid
Figure 1.1 Anaerobic part of biological oxidation.