Page 35 - Biofuels Refining and Performance
P. 35
18 Chapter One
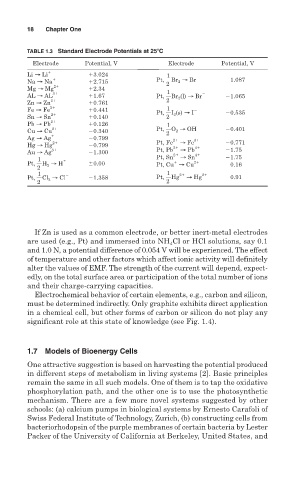
TABLE 1.3 Standard Electrode Potentials at 25 C
Electrode Potential, V Electrode Potential, V
Li → Li 3.024 1
Na → Na 2.715 Pt, Br 2 → Br 1.087
2
Mg → Mg 2 2.34 1
AL → AL 3 1.67 Pt, Br 2 (l) → Br 1.065
Zn → Zn 2 0.761 2
1
Fe → Fe 2 0.441 Pt, I 2 (s) → I 0.535
Sn → Sn 2 0.140 2
Pb → Pb 2 0.126 1
Cu → Cu 2 0.340 Pt, O 2 → OH 0.401
2
Ag → Ag 0.799 2 3
Hg → Hg 2 0.799 Pt, Fe 2 → Fe 4 0.771
Au → Ag 3 1.300 Pt, Pb 2 → Pb 4 1.75
1 Pt, Sn → Sn 1.75
Pt, H 2 → H 0.00 Pt, Cu → Cu 2 0.16
2
1 1 2 2
Pt, Cl 2 → Cl 1.358 Pt, Hg → Hg 0.91
2 2
If Zn is used as a common electrode, or better inert-metal electrodes
are used (e.g., Pt) and immersed into NH Cl or HCl solutions, say 0.1
4
and 1.0 N, a potential difference of 0.054 V will be experienced. The effect
of temperature and other factors which affect ionic activity will definitely
alter the values of EMF. The strength of the current will depend, expect-
edly, on the total surface area or participation of the total number of ions
and their charge-carrying capacities.
Electrochemical behavior of certain elements, e.g., carbon and silicon,
must be determined indirectly. Only graphite exhibits direct application
in a chemical cell, but other forms of carbon or silicon do not play any
significant role at this state of knowledge (see Fig. 1.4).
1.7 Models of Bioenergy Cells
One attractive suggestion is based on harvesting the potential produced
in different steps of metabolism in living systems [2]. Basic principles
remain the same in all such models. One of them is to tap the oxidative
phosphorylation path, and the other one is to use the photosynthetic
mechanism. There are a few more novel systems suggested by other
schools: (a) calcium pumps in biological systems by Ernesto Carafoli of
Swiss Federal Institute of Technology, Zurich, (b) constructing cells from
bacteriorhodopsin of the purple membranes of certain bacteria by Lester
Packer of the University of California at Berkeley, United States, and