Page 34 - Biofuels Refining and Performance
P. 34
Energy and Its Biological Resources 17
Zn Cu Zn Cu Cu Zn
Zn 2+ Cu 2+ Zn 2+ Cu 2+ Zn +2 Cu +2
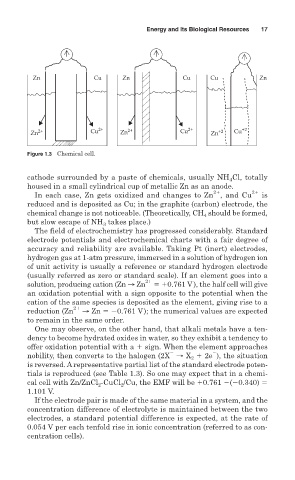
Figure 1.3 Chemical cell.
cathode surrounded by a paste of chemicals, usually NH Cl, totally
4
housed in a small cylindrical cup of metallic Zn as an anode.
2 2
In each case, Zn gets oxidized and changes to Zn , and Cu is
reduced and is deposited as Cu; in the graphite (carbon) electrode, the
chemical change is not noticeable. (Theoretically, CH should be formed,
4
but slow escape of NH takes place.)
3
The field of electrochemistry has progressed considerably. Standard
electrode potentials and electrochemical charts with a fair degree of
accuracy and reliability are available. Taking Pt (inert) electrodes,
hydrogen gas at 1-atm pressure, immersed in a solution of hydrogen ion
of unit activity is usually a reference or standard hydrogen electrode
(usually referred as zero or standard scale). If an element goes into a
2
solution, producing cation (Zn → Zn 0.761 V), the half cell will give
an oxidation potential with a sign opposite to the potential when the
cation of the same species is deposited as the element, giving rise to a
2
reduction (Zn → Zn 0.761 V); the numerical values are expected
to remain in the same order.
One may observe, on the other hand, that alkali metals have a ten-
dency to become hydrated oxides in water, so they exhibit a tendency to
offer oxidation potential with a sign. When the element approaches
nobility, then converts to the halogen (2X → X 2e ), the situation
2
is reversed. A representative partial list of the standard electrode poten-
tials is reproduced (see Table 1.3). So one may expect that in a chemi-
-CuCl /Cu, the EMF will be 0.761 ( 0.340)
cal cell with Zn/ZnCl 2 2
1.101 V.
If the electrode pair is made of the same material in a system, and the
concentration difference of electrolyte is maintained between the two
electrodes, a standard potential difference is expected, at the rate of
0.054 V per each tenfold rise in ionic concentration (referred to as con-
centration cells).