Page 31 - Biofuels Refining and Performance
P. 31
14 Chapter One
The percentage efficiency figures raise doubt about the interpreta-
tion. Such efficiency is never achieved by a man-made machine but
biological systems can. If we accept the lower figures with a margin,
we are conserving no less than 25% of our expenditure in the form
of provident fund energy, even under sudden stress, i.e., anaerobic
conditions.
Let us look at the situation when a reduced coenzyme is regenerated
or oxidized (brief and simplified):
1
ATP ATP ATP
O 2
2
NADH (H ) ⎯→ FAD ⎯→ Cytochrome ⎯→ Cytochrome ⎯→ H O
2
Stoichiometrically,
1
CoIH(H ) O 3ADP 3H PO → CoI 3ATP 4H O
2
2
4
2
2
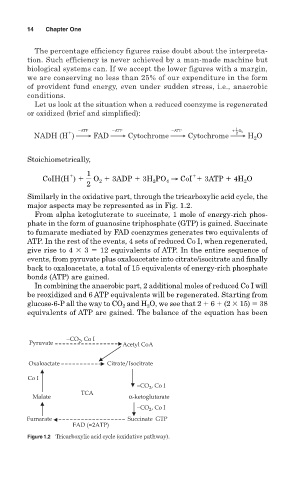
Similarly in the oxidative part, through the tricarboxylic acid cycle, the
major aspects may be represented as in Fig. 1.2.
From alpha ketogluterate to succinate, 1 mole of energy-rich phos-
phate in the form of guanosine triphosphate (GTP) is gained. Succinate
to fumarate mediated by FAD coenzymes generates two equivalents of
ATP. In the rest of the events, 4 sets of reduced Co I, when regenerated,
give rise to 4 3 12 equivalents of ATP. In the entire sequence of
events, from pyruvate plus oxaloacetate into citrate/isocitrate and finally
back to oxaloacetate, a total of 15 equivalents of energy-rich phosphate
bonds (ATP) are gained.
In combining the anaerobic part, 2 additional moles of reduced Co I will
be reoxidized and 6 ATP equivalents will be regenerated. Starting from
glucose-6-P all the way to CO and H O, we see that 2 6 (2 15) 38
2
2
equivalents of ATP are gained. The balance of the equation has been
−CO , Co I
2
Pyruvate Acetyl CoA
Oxaloactate Citrate/Isocitrate
Co I
−CO , Co I
2
TCA
Malate α-ketoglutarate
−CO , Co I
2
Fumarate Succinate GTP
FAD (=2ATP)
Figure 1.2 Tricarboxylic acid cycle (oxidative pathway).