Page 36 - Biofuels Refining and Performance
P. 36
Energy and Its Biological Resources 19
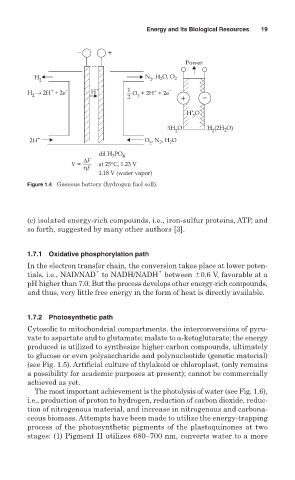
− +
Power
H N , H O, O 2
2
2 2
+
+
H → 2H + 2e − H + 1 O + 2H + 2e −
2 2 2 + −
+
H O
3
3H O H (2H O)
2 2 2
2H + O , N , H O
2
2
2
dil H PO 4
3
∆F
V = at 25°C, 1.23 V
ηF
1.18 V (water vapor)
Figure 1.4 Gaseous battery (hydrogen fuel cell).
(c) isolated energy-rich compounds, i.e., iron-sulfur proteins, ATP, and
so forth, suggested by many other authors [3].
1.7.1 Oxidative phosphorylation path
In the electron transfer chain, the conversion takes place at lower poten-
tials, i.e., NAD/NAD to NADH/NADH between 0.6 V, favorable at a
pH higher than 7.0. But the process develops other energy-rich compounds,
and thus, very little free energy in the form of heat is directly available.
1.7.2 Photosynthetic path
Cytosolic to mitochondrial compartments, the interconversions of pyru-
vate to aspartate and to glutamate; malate to -ketoglutarate; the energy
produced is utilized to synthesize higher carbon compounds, ultimately
to glucose or even polysaccharide and polynucleotide (genetic material)
(see Fig. 1.5). Artificial culture of thylakoid or chloroplast, (only remains
a possibility for academic purposes at present); cannot be commercially
achieved as yet.
The most important achievement is the photolysis of water (see Fig. 1.6),
i.e., production of proton to hydrogen, reduction of carbon dioxide, reduc-
tion of nitrogenous material, and increase in nitrogenous and carbona-
ceous biomass. Attempts have been made to utilize the energy-trapping
process of the photosynthetic pigments of the plastoquinones at two
stages: (1) Pigment II utilizes 680–700 nm, converts water to a more